-
PDF
- Split View
-
Views
-
Cite
Cite
Emer F. Cahill, Helen Kennelly, Fiona Carty, Bernard P. Mahon, Karen English, Hepatocyte Growth Factor Is Required for Mesenchymal Stromal Cell Protection Against Bleomycin-Induced Pulmonary Fibrosis, Stem Cells Translational Medicine, Volume 5, Issue 10, October 2016, Pages 1307–1318, https://doi.org/10.5966/sctm.2015-0337
Close - Share Icon Share
Abstract
The incidence of idiopathic pulmonary fibrosis is on the rise and existing treatments have failed to halt or reverse disease progression. Mesenchymal stromal cells (MSCs) have potent cytoprotective effects, can promote tissue repair, and have demonstrated efficacy in a range of fibrotic lung diseases; however, the exact mechanisms of action remain to be elucidated. Chemical antagonists and short hairpin RNA knockdown were used to identify the mechanisms of action used by MSCs in promoting wound healing, proliferation, and inhibiting apoptosis. Using the bleomycin induced fibrosis model, the protective effects of early or late MSC administration were examined. The role for hepatocyte growth factor (HGF) in MSC protection against bleomycin lung injury was examined using HGF knockdown MSC. Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling assay was performed on ex vivo lung sections to examine the effects of MSC on apoptosis. MSC conditioned media (CM) enhanced wound closure and inhibited apoptosis of pulmonary cells in vitro. HGF was required for MSC CM enhancement of epithelial cell proliferation and inhibition of apoptosis. In contrast, MSC required COX-2 for CM to inhibit fibroblast proliferation. In a murine model, early administration of MSC protected against bleomycin induced lung fibrosis and correlated with reduced levels of the proinflammatory cytokine interleukin-1β, reduced levels of apoptosis, and significantly increased levels of HGF. These protective effects were in part mediated by MSC derived HGF as HGF knockdown MSC were unable to protect against fibrosis in vivo. These findings delineate the mechanisms of MSC protection in a preclinical model of fibrotic lung disease.
The mechanisms used by mesenchymal stromal cells (MSCs) in mediating protective effects in chronic models of lung disease are not understood and remain to be elucidated. These findings from in vitro studies highlight an important role for the MSC-derived soluble factors hepatocyte growth factor (HGF) and prostaglandin E2 in promoting wound healing and inhibiting apoptosis. Furthermore, this study translates these findings demonstrating an important role for HGF in the protective effects mediated by MSC in vivo in the bleomycin model. These findings support a targeted approach to enhancing MSC therapy for fibrotic disease and highlight the importance of timing of MSC therapy.
Introduction
Pulmonary fibrosis is a chronic disease associated with remodeling of the lung architecture and excessive deposition of activated fibroblasts in parenchymal tissue. This leads to formation of scar tissue, which becomes a permanent feature once formed. Fibrosis can arise from chronic stimuli; however, many cases are classified as idiopathic. The disease is life threatening with limited treatment options, often necessitating organ transplant. The exact cause of pulmonary fibrosis is as yet unknown but ultimately results from aberrant wound healing.
Normal wound healing can be divided into three principle stages: inflammation, proliferation, and repair. In the case of pulmonary fibrosis, the initial injury can come in the form of a chemical, biological, or physical insult, and more often than not, the stimulus is chronic [1]. Irrespective of the source, damage to the lung epithelium results in exposure of endothelium and basement membrane proteins leading to the recruitment and activation of innate and adaptive immune cells [2]. The final phase of wound healing, repair, is a delicate balance between the degradation of scar tissue and the generation of new epithelium and, depending on the type of wound, can take years [3]. In vivo studies on fibrosis have relied predominantly on the bleomycin induced animal model of pulmonary fibrosis. The damage in this model is caused by bleomycin-induced reactive oxygen species and cleavage of DNA strands [4].
Mesenchymal stromal (stem) cells (MSCs) can inhibit inflammation and regulate immune responses in vitro and in vivo [5–8]. In addition, MSCs can differentiate into a number of lineages including bone, fat, and cartilage. However, it is becoming apparent that the protective effects associated with MSC therapy are based less on in situ differentiation abilities and more on paracrine or trophic factors [9]. MSCs can home to sites of injury and induce repair through the release of trophic factors, such as cytokines [10]. The importance of chemotaxis, engraftment, [11, 12] and cytokine production [13–15] in MSC protection against bleomycin induced pathology has been demonstrated by a number of preclinical studies [12–13, 14–16]; however, much remains unknown about how MSCs function. MSCs are known to mediate potent anti-inflammatory effects in the context of lung inflammation. For example, MSCs can promote the polarization of anti-inflammatory M2 macrophages [16] and regulatory T cells [5, 17], and they directly inhibit effector T cells [18]. Recently, allogeneic human MSCs have been shown to be safe in a clinical trial in chronic obstructive pulmonary disease with an early significant decrease in circulating levels of C-reactive protein [19]. The increasing use of allogeneic MSCs instead of autologous MSCs is an important factor in a broadly applicable off-the-shelf therapy and supports the use of allogeneic MSCs in preclinical studies where appropriate.
In the setting of bleomycin-induced fibrosis, the major cells involved are fibroblasts and epithelial cells. The effects of MSCs and MSC-derived trophic factors on these cell types in wound healing, apoptosis, and fibrosis are not understood.
Hepatocyte growth factor (HGF) is paracrine growth factor involved in growth, proliferation, and migration of epithelial and endothelial cells, its primary function being tissue repair [20]. HGF treatment has been shown to have a positive effect in the bleomycin model, demonstrating a reduction in pathology and collagen deposition following immediate and delayed administration [21], as well as encouraging the proliferation of epithelial cells both in vitro and in vivo [22]. MSCs can be induced to secrete HGF following inflammatory stimulation [22, 23].
This study aimed to identify the mechanisms of action of allogeneic MSC protective effects on pulmonary cells in vitro, influencing the proliferation and migration of fibroblasts and alveolar epithelial cells. These effects were mediated by soluble factors released by MSCs. In particular, MSC secretion of HGF played a key role in MSC promotion of wound healing and prevention of apoptosis. Using short hairpin RNA (shRNA) technology, the relevance of HGF secretion by MSC in support of wound healing was demonstrated, supporting a role for allogeneic MSC as a cell therapy in the early stages of pulmonary fibrosis.
Materials and Methods
Animals
Six- to 8-week-old female BALB/c, or C57BL/6 mice (Envigo, Cambridge, U.K., http://www.envigo.com.), were used for cell based experiments under the guidelines of the Irish Department of Health and the approval of the research ethics committee of Maynooth University. Mice were maintained according to the regulations of the Irish Department of Health and the institutional research ethics committee at Maynooth University. C57BL/6 mice were chosen at random, weighed, and administered 4 U/kg of bleomycin by intranasal instillation. The mice were lightly anesthetized with gaseous isofluorane, and 20 µl of bleomycin diluted in phosphate-buffered saline (PBS) was administered to the nostrils allowing the solution to be inhaled into the lungs. Control groups were given 20 µl of PBS. Allogeneic BALB/c MSC (5 × 104/g) were administered by intravenous injection to the tail vein using a 27-gauge needle and a 1-ml syringe either 6–8 hours after bleomycin administration or 9 days later, and PBS was injected as a negative control.
Histopathology
On day 28, lungs were harvested, fixed, and paraffin embedded followed by sectioning and staining with hematoxylin and eosin (H&E) (Sigma-Aldrich, St. Louis, MO, https://www.sigmaaldrich.com) and Masson's trichrome stain (Sigma-Aldrich). For H&E staining tissue sections were stained in Harris hematoxylin solution and followed by counter staining with eosin. For Masson's trichrome stain, tissue was stained with Harris hematoxylin solution (nuclei), scarlet-acid fuchsin (muscle), and aniline blue (connective tissue). Slides were then mounted with DPX Mountant (Sigma-Aldrich) and airway pathology was examined in a blinded fashion under a light microscope. At least five fields of view were examined in five sections from five different mice per group. Blinded analysis was then verified by two other researchers. A semiquantitative scoring system was used to assess collagen deposition and changes in lung architecture [23].
Isolation and Characterization of Bone Marrow-Derived MSCs
Murine MSCs were isolated from the bone marrow of BALB/c mice as previously described [5]. Briefly, following the isolation of bone marrow, MSCs were cultured in complete Roswell Park Memorial Institute (RPMI), supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific, Minneapolis, MN, https://www.thermofisher.com), 10% (vol/vol) equine serum (HyClone Laboratories, GE Healthcare Life Sciences, Logan, UT, https://promo.gelifesciences.com/gl/hyclone), 1% (vol/vol) penicillin/streptomycin (Gibco), and 1% (vol/vol) l-glutamine (Gibco) for 28 days, replacing with fresh media every 3–4 days. At passage 2, MSCs were expanded in complete α-minimum essential medium (CEM) supplemented with 10% (vol/vol) heat-inactivated FBS, 10% (vol/vol) equine serum, 1% (vol/vol) penicillin/streptomycin, and 1% (vol/vol) l-glutamine. MSCs were analyzed for the expression of a variety of fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated surface markers including Sca-1, major histocompatibility complex (MHC) class I, MHC class II, CD11b, CD11c, CD34, CD44, CD45, CD80, CD86, CD90, CD105, CD106, and CD117 (eBioscience, Hatfield, U.K., http://www.ebioscience.com) by flow cytometry (BD FACSCalibur and CellQuest software, BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) at every passage. The differentiation capacity of MSCs following the addition of differentiation media [5] into osteocytes, adipocytes, and chondrocytes were analyzed at every passage as previously described. MSCs used in these studies expressed MHC class I, Sca-1, CD44, and CD106 and differentiated into all three lineages mentioned above. The ability of MSCs to suppress allogeneic and mitogen-driven proliferation was also assessed at each passage. MSCs were used between passages 3 and 10.
Isolation and Characterization of Primary Lung Fibroblasts
Primary lung fibroblasts were isolated from female C57BL/6 mice. The lungs were washed, digested using trypsin (Gibco), and diced before incubation with DNase I solution (Sigma-Aldrich). The cell suspension was centrifuged and resuspended in DNase II solution (Sigma-Aldrich), followed by centrifugation and resuspension in M199:Hams/F12 supplemented with 1% (vol/vol) penicillin/streptomycin and 1% (vol/vol) l-glutamine. The cell suspension was seeded into a 10-cm nontissue grade petri dish and incubated for 1 hour at 37°C, during which time fibroblasts and macrophages adhered to the surface of the petri dish. Nonadherent cells were removed and the adherent cells were allowed to reach confluence in high-glucose Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich) supplemented with 10% (vol/vol) heat inactivated FBS, 1% (vol/vol) penicillin/streptomycin, 1% (vol/vol) l-glutamine, 1 ng/ml β-FGF (Peprotech, London, United Kingdom, https://www.peprotech.com). Fibroblasts were isolated from macrophages through selective passaging with 2-minute trypsin washes. At passage 2, the cells were characterized and stocks frozen in liquid nitrogen. Primary fibroblasts were used in experiments up to passage 5.
Short Hairpin RNA
Five HGF shRNA plasmids were expressed in the lentiviral vector pGIPZ (Open Biosystems, GE Healthcare Life Sciences, http://dharmacon.gelifesciences.com/about-us/about open biosystems). HGF plasmids were transfected into HEK293T cells along with the packaging plasmid trans-lentiviral packaging mix (Open Biosystems) using Gene Juice (Merck Millipore, Darmstadt, Germany, http://www.merckmillipore.com). HEK293T cells were cultured in high-glucose DMEM (Sigma-Aldrich) supplemented with 10% (vol/vol) heat inactivated FBS, 1% (vol/vol) penicillin/streptomycin, and 1% (vol/vol) l-glutamine, and during transfection they were cultured in serum-free DMEM without antibiotics. After 24-hour transfection, they were cultured in medium containing 30% FBS. Forty-eight hours after transfection, the supernatant containing the HGF shRNA lentivirus was collected. BALB/c MSCs were transduced with the viral medium for 72 hours followed by positive selection using 4 µg/ml puromycin. Expression and production of HGF were examined by real-time polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, https://www.rndsystems.com) according to the manufacturer's instructions to ensure stable knockdown following subcloning.
Generation of MSC-Conditioned Medium
MSCs (1 × 105 cells per 2 ml) were seeded in normal CEM and allowed to adhere for 6 hours. MSCs were washed with PBS and LA4, or fibroblast media was added. LA4 medium; Dulbecco's modified Eagle's medium nutrient mixture F12 (DMEM F12) (Sigma-Aldrich) supplemented with 10% (vol/vol) heat inactivated FBS, 1% (vol/vol) penicillin/streptomycin, and 1% (vol/vol) l-glutamine. Fibroblast medium; as described earlier. The conditioned media (CM) were collected after 24 hours and concentrated using Amicon Ultra Centrifugal filters (Sigma-Aldrich). The resulting CM -20 μl concentrate derived from 1 × 105 MSCs during a 24-hour period was then added to the medium in the assays described later.
Coculture Experiments: MSCs, Fibroblasts, and LA4 Cells
Scratch Assay
The base of a six-well tissue culture plate (Sarstedt, Wexford, Ireland, https://www.sarstedt.com) was scored with three parallel lines using a safety blade. Fibroblast or LA4 (lung alveolar epithelial cells) cells were seeded at a concentration of 1 × 105 cells per well and allowed to form a confluent monolayer overnight in fibroblast or LA4 medium. Using a P200 pipette tip (Sarstedt), a line was scratched down the center of the well perpendicular to the three lines on the base of the plate. The wells were washed with warm PBS to remove floating cells, and fresh medium was added to all the wells. CM collected from MSC seeded in the appropriate media were concentrated using Amicon Ultra Centrifugal filters, and the resulting protein-enriched fraction (20 μl) was added to designated wells. The width of the scratch was measured using Photoshop (Adobe, San Jose, CA, https://www.adobe.com) from images taken using an inverted light microscope, and the lines scored on the bottom of the wells were used as an alignment guide. The wells were observed daily. Once the first well showed a full closure of the scratch, cells were fixed with ice cold methanol for 5 minutes and were stained with crystal violet for 10 minutes. The width of the closure was measured in all cultures from the same position and the results were graphed as a percentage of closure when compared with the positive control.
Proliferation Assay
The confluent monolayer of LA4 or fibroblast cells was scratched with a P1000 pipette tip and washed with PBS to remove detached cells. Media collected from MSC (conditioned media) was added to the designated wells. CEM was added to the control wells. Tritiated thymidine (3H-thymidine) (Amersham Biosciences, GE Life Sciences) at 0.5 µCi/ml was added to each well and incubated at 37°C 5% carbon dioxide. Cultures were harvested after 3 days by removing the media and washing each well three times with PBS. The cells were lysed using 500 µl of 1% sodium dodecyl sulfate (Sigma-Aldrich) and the lysate was transferred to 3-ml plastic counting tubes. Two milliliters of β-scintillation fluid (UltmaGold, Wallace-PerkinElmer, Waltham, MA, http://www.perkinelmer.com) was added to each tube, vortexed, and placed in a 24-well cassette for reading. 3H-Thymidine incorporation was quantified by a β-scintillation counter (1450 Microbeta Liquid Scintillation Counter; Wallace-PerkinElmer) and results were expressed in counts per minute.
Apoptosis Assay
Apoptosis was induced in LA4 cells by addition of cisplatin at 4 μg/ml and incubated for 24 hours. MSC CM or control media were added to test wells. Conditioned media were collected from nontransduced MSCs, HGF knockdown MSCs, and nonsilencing control MSCs and were added with cisplatin to the appropriate wells. After 24 hours, apoptosis was measured by double staining of annexin V and propidium iodide (PI) (eBioscience) and analyzed by flow cytometry. The percentage of early apoptotic cells (lower right quadrant annexin V+ PI–) was determined and graphed for comparison.
Myofibroblast Differentiation Assay
Fibroblasts were cultured at 2 × 105 cells per well of a six-well plate. The cells were stimulated with 50 ng/ml of recombinant mouse TGF-β1 (R&D Systems), with the control well receiving no stimulant. MSC CM was added to a further well containing TGF-β1 and was cultured for 24 hours. The cells were collected in TRIzol Reagent (Thermo Fisher) and were examined for expression of mouse α smooth muscle actin (α-SMA) by quantitative real-time PCR.
Reverse Transcription-PCR and Real-Time PCR Analysis
Reverse transcription (RT)-PCR analysis was used to determine the mRNA expression levels of mouse aggrecan and collagen IIA in differentiated MSCs and HGF knockdown MSC. Real-time PCR was used to determine the mRNA expression of α-SMA in resting and stimulated fibroblasts and expression of mouse tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), HGF, and TGF-β in lungs tissue taken from the bleomycin model. TRI Reagent (Thermo Fisher) was used to isolate total RNA and samples were reverse transcribed to cDNA using a Tetro cDNA synthesis kit (Biloine, Taunton, MA, http://www.bioline.com). RT-PCR analysis was performed using a MyTaq DNA polymerase kit (Bioline). Real-time PCR analysis was performed using SYBR Green reagent (Sigma-Aldrich) and specific primers were used to determine gene expression. The following PCR predesigned primers (Sigma-Aldrich) were used: α-SMA: 5′-3′ CATCTTTCATTGGGATGGAG and 3′-5′ TTAGCATAGAGATCCTTCCTG; TNF-α: 5′-3′ GGATGAGAAGTTCCCAAAT and 3′-5′ TGAGAAGATGATCTGAGTG; IL-1β: 5′-3′ GGATGATGATGATAACCTGC and 3′-5′ CATGGAGAATATCACTTGTTGG; HGF: 5′-3′ CAAATGCAAGGACCTTAGA and 3′-5′ CTTCTTTTGGATAAGTTGCC; TGF-β: 5′-3′ GGATACCAACTATTGCTTCAG and 3′-5′ TGTCCAGGCTCCAAATATAG. Glyceraldehyde-3-phosphate dehydrogenase expression was used as a housekeeping gene using the following primers: 5′-3′ GCACAGTCAAGGCCGAGAAT and 3′-5′ GCCTTCTCCATGGTGGTGAA.
Statistics
Statistical analysis was performed using GraphPad Prism software (GraphPad, San Diego, CA, http://www.graphpad.com/company). The Student's paired t test was used when statistical analysis was required between two experimental groups. One-way analysis of variance was used to test for statistical significance of differences when multiple experimental groups were compared. Data are presented as the mean ± the standard error of the mean (SEM). p values of p < .05, p < .01, or p < .001 were considered statistically significant.
Results
MSC-Conditioned Media Enhanced Wound Healing In Vitro
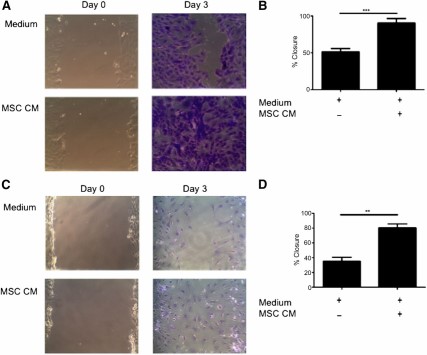
Wound healing is a complex system involving multiple cell types and signals with an unknown trigger leading to deregulation and induction of fibrosis, making it difficult to replicate on the bench. A simplified in vitro model of wound healing (scratch assay) was used to determine whether MSCs were having an effect on this system. The scratch assay was used to examine MSC trophic effects on primary murine fibroblasts and LA4 cells. The addition of MSC CM to scratch cultures significantly increased percentage wound closure at day 3 in both fibroblasts (Figs. 1A and 1B) and epithelial cells (Figs. 1C and 1D), when compared with wells receiving control medium. These data demonstrate soluble factors produced by MSC significantly promote wound closure by epithelial cells and fibroblasts.
MSC CM enhances wound closure of murine pulmonary cells in vitro. (A): Representative images (magnification × 100) and (B): Quantification of scratch assay examining the migration of primary murine lung fibroblasts in the presence or absence of MSC CM for 0 and 3 days. Representative images (magnification × 100) (C) and quantification (D) of scratch assay examining migration of murine alveolar epithelial cells (LA4) in the presence of MSC CM at time 0 and after 3 days. Images are representative of three experiments and bar graph data are presented as mean ± standard error of the mean of n = 3. ∗∗, p < .01; ∗∗∗, p < .001. Abbreviations: CM, conditioned media; MSC, mesenchymal stromal cell.
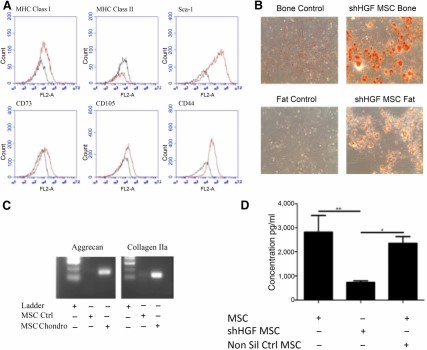
HGF is known to enhance lung epithelial cell; CM, conditioned medium proliferation and is an important soluble mediator released by MSCs [24]. Lentiviral shRNA techniques were used to create a stable line of HGF knockdown MSCs (Fig. 2). Characterization using surface marker expression (Fig. 2A) and differentiation assays (Figs. 2B and 2C) demonstrated that shHGF MSCs (shRNA HGF knockdown MSCs) maintain the characteristics of MSCs. Production of HGF was significantly reduced in shHGF MSCs but not in nonsilencing control MSCs (Fig. 2D).
shHGF MSCs maintain the characteristics of MSCs. HGF knockdown MSCs were tested for (A): surface marker expression of MHC class I, MHC class II, Sca-1, CD73, CD105, and CD44 by flow cytometry; (B): Differentiation into bone and fat cells (magnification × 100); and (C): Chondrocytes. shHGF MSCs retained the characteristics of MSCs. (D): HGF knockdown MSC conditioned media (CM), nontransduced MSC CM, and nonsilencing control MSC CM were examined for levels of HGF by enzyme-linked immunosorbent assay. Data are representative of three experiments for A–C and n = 3 for D. ∗, p < .05; ∗∗, p < .01. Abbreviations: Chondro, chondrocytes; CM, conditioned medium; Ctrl, control; HGF, hepatocyte growth factor; MHC, major histocompatibility complex; MSC, mesenchymal stromal cell; Non Sil Ctrl, nonsilencing control; shHGF, shRNA HGF knockdown.
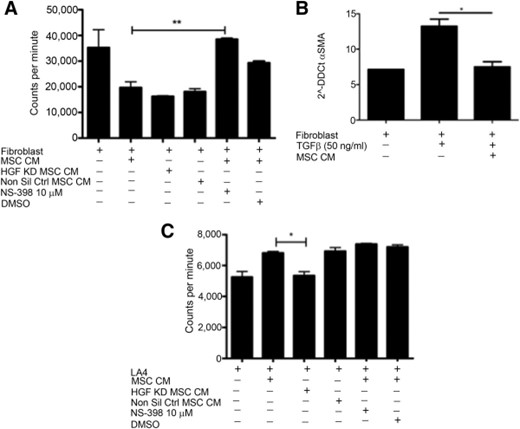
To investigate whether MSC CM enhanced wound closure through the promotion of migration or proliferation of these cells, 3H-thymidine was added to the cultures. The addition of MSC CM to scratched fibroblast cultures not only prevented an increase in proliferation but reduced the proliferation to levels significantly lower than basal division (Fig. 3A). Excessive proliferation of fibroblasts is a hallmark of fibrosis, and although fibroblasts play a vital role in wound healing, their accumulation is associated with pathogenesis. Prostaglandin E2 (PGE2) is a key signaling molecule involved in fibroblast homeostasis [25–27] and is known to be secreted by MSCs [28]. To determine the potential role for PGE2 signaling in MSC inhibition of fibroblast proliferation, a specific inhibitor of COX-2 (an enzyme required for the production of PGE2) was added to MSC cultures. Addition of NS-398 to fibroblast scratch assays containing 3H-thymidine significantly prevented MSC CM from inhibiting the proliferation of fibroblasts (Fig. 3A), whereas knocking down HGF had no impact on MSC CM capacity to inhibit fibroblast proliferation (Fig. 3A). This data show that PGE2 plays an important role in MSC inhibition of fibroblast proliferation. The activation of fibroblasts and differentiation into pathogenic myofibroblasts plays an important role in fibrosis [29]. To examine the influence of MSC CM on fibroblast activation, the expression of α-SMA was examined by RT-PCR. MSC CM significantly reduced the expression of α-SMA in fibroblasts following stimulation with TGF-β (Fig. 3B). In contrast to the suppressive effects on fibroblasts, MSC CM had differential effects on airway epithelial cells. When cultured with LA4 cells, MSC CM significantly increased the proliferation of the epithelial cells (Fig. 3C).
MSCs enhance proliferation of LA4 cells and suppress proliferation and differentiation of fibroblasts. (A): Proliferation of fibroblasts was determined by measuring titrated thymidine uptake. MSC CM inhibition of fibroblast proliferation was negated with the addition of 10 µM NS398 but not by the vehicle control DMSO or by HGF KD MSC CM. (B): TGF-β driven differentiation of fibroblasts to myofibroblasts was inhibited by MSC CM. (C): MSC CM promotion of LA4 proliferation was lost when HGF KD MSC CM was used but NS398 had no impact. n = 3. ∗, p < .05; ∗∗, p < .01. Abbreviations: CM, conditioned media; DMSO, dimethyl sulfoxide; HFG, hepatocyte growth factor; KD, knockdown; LA4, murine alveolar epithelial cell; MSC, mesenchymal stromal cell; Non Sil Ctrl, nonsilencing control; α-SMA, α smooth muscle actin; TGF-ß, transforming growth factor beta.
CM collected from MSC was used in culture with LA4 cells to determine the importance of HGF and PGE2 in MSC CM-induced proliferation of epithelial cells. Inhibition of the COX-2 pathway had no impact on MSC CM promotion of epithelial cell proliferation. Cultures with HGF knockdown MSC CM showed significantly lower levels of proliferation compared with those containing nontransduced or nonsilencing control MSC CM (Fig. 3C).
MSC CM Reduction in Epithelial Cell Apoptosis Depends on HGF Signaling
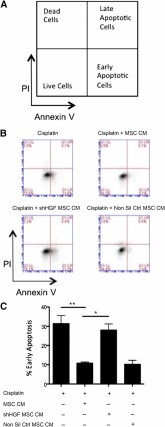
Following the observation that MSC conditioned media enhanced in vitro wound healing and epithelial proliferation, further in vitro work was carried out to examine the potential to inhibit epithelial apoptosis. Increased epithelial apoptosis is observed alongside the collagen deposition that results in the significant tissue scarring observed in the lungs during pulmonary fibrosis. Using LA4 epithelial cells, apoptosis was induced through the addition of cisplatin (a chemotherapeutic agent known to induce apoptosis [30]). Cells were cultured for 24 hours either in media alone or MSC CM. Flow cytometric measurement of annexin V and PI demonstrated that cisplatin induced LA4 cells to undergo apoptosis. The percentage of early apoptotic cells (Annexin V+ PI-) (gating strategy shown in Fig. 4A) was significantly reduced with the addition of MSC CM (Figs. 4B and 4C). This demonstrated that the trophic factors produced by MSCs possess antiapoptotic properties for epithelial cells. To investigate if this antiapoptotic effect was HGF dependent, HGF knockdown MSC CM and nonsilencing control CM were added to the LA4 cells at the same time as cisplatin. The antiapoptotic effect observed with MSC CM was significantly reduced in LA4 cells cultured with MSC HGF knockdown CM but not the nonsilencing control CM (Figs. 4B and 4C). These data demonstrate that the soluble factors produced by MSCs have an antiapoptotic effect on epithelial cells in vitro and that this effect is largely dependent on HGF signaling.
MSC CM suppresses apoptosis of lung epithelial cells. (A): Gating strategy; (B): Representative FACS plots; and (C): Quantification of apoptosis assay examining the ability of MSC CM to inhibit alveolar epithelial apoptosis. Apoptosis was measured by double staining of annexin V and PI and measured by flow cytometry. MSC CM significantly reduced epithelial apoptosis induced by cisplatin. This effect was lost when CM from HGF knockdown cells was used. CM from nonsilencing controls caused no significant difference. Data are representative of three experiments in B and n = 3 for C. Bar graph presents the % of early apoptotic cells (lower right quadrant: annexin V+ PI-) as mean ± standard error of the mean. ∗, p < .05; ∗∗, p < .01. Abbreviations: CM, conditioned media; HGF, hepatocyte growth factor; MSC, mesenchymal stromal cell; Non Sil Ctrl, nonsilencing control; PI, propidium iodide; shHGF, shRNA HGF knockdown.
Early but Not Delayed Administration of MSCs Protects Against Bleomycin-Induced Lung Fibrosis
To determine whether the positive effects observed with MSC in vitro translated in vivo, the bleomycin murine model of lung fibrosis was exploited. A time course study examining a range of fibrotic and inflammatory genes showed the onset of fibrosis occurs at approximately day 15, with inflammation beginning 6 days earlier, allowing further studies to discriminate between anti-inflammatory and antifibrotic responses (supplemental online Fig. 1).
This study sought to differentiate between MSCs as an anti-inflammatory treatment and an antifibrotic therapeutic; therefore, administration of MSCs was delayed until the onset of fibrosis. Based on the results from the time course study, MSCs were given at day 0 and day 9. Lungs were collected on day 21 for PCR analysis and day 28 for histological examination.
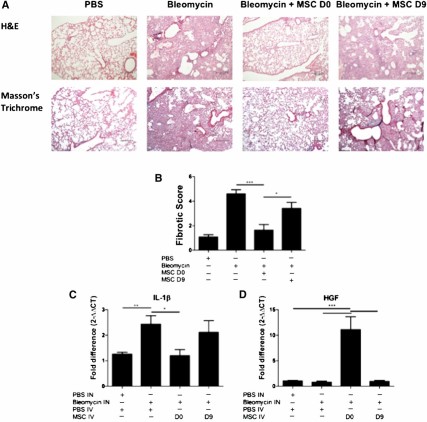
Bleomycin caused a significant increase in collagen deposition and fibrotic tissue, in comparison with the PBS control lungs (Fig. 5A). Treatment with MSC on day 0 reduced the amount of collagen detected in the lungs; however, administration of MSCs on day 9 did not demonstrate the same ability to reduce this collagen deposition (Fig. 5A). The tissue score shows that bleomycin caused significant fibrotic changes in the lungs. This fibrosis was significantly reduced with MSC day 0 treatment. Although MSC day 9 treatment resulted in some reduction in fibrosis, there was a significant difference between the two timings of MSC delivery, again highlighting the differing effects of MSCs administered at different time points (Fig. 5B). There were also statistically higher levels of the proinflammatory cytokine IL-1β at day 21 in bleomycin-treated lungs compared with PBS. Consistent with the protective effect observed in the lung tissue, early administration of MSC on day 0 but not on day 9 significantly decreased the mRNA expression of IL-1β in the lungs (Fig. 5C). The protective effect mediated by early administration of MSCs was associated with significantly elevated levels of the cytoprotective factor HGF (Fig. 5D).
Early administration of MSCs protects lungs from bleomycin-induced fibrosis. Lungs were harvested on D28 of the bleomycin model for histological assessment. (A): Representative images of H&E (upper panel) and trichrome-stained (lower panel) lung sections (10×) show collagen deposition and alveolar damage. Scale bar = 200 μm. (B): Fibrotic score was significantly reduced when MSCs were administered on D0 but not on D9. (C): mRNA expression of IL-1β was measured in the lungs using qPCR on D21 of the bleomycin study. IL-1β mRNA expression was reduced when MSCs were administered on D0 but not day D9. (D): HGF mRNA expression was measured in the lungs using quantitative polymerase chain reaction. HGF mRNA expression was enhanced when MSCs were administered on D0 but not on D9. n = 5. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001. Abbreviations: D, day; H&E, hematoxylin and eosin; HGF, hepatocyte growth factor; IL-1β, interleukin-1β; IN, intranasal; IV, intravenous; MSC, mesenchymal stromal cell; PBS, phosphate-buffered saline.
HGF Knockdown in MSCs Abrogates the Protective Effects Observed With Administration at Day 0
Based on our in vitro findings demonstrating the important role for MSC-derived HGF in the promotion of wound healing and the correlation between increased HGF expression in vivo and MSC therapeutic efficacy, we examined the role of HGF in MSC protection in the bleomycin model.
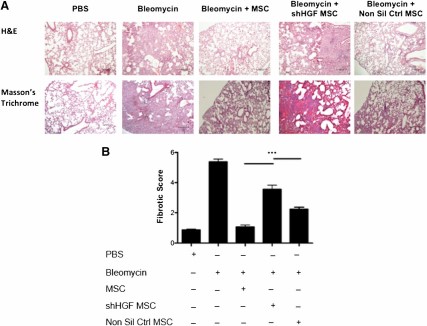
HGF knockdown MSCs were directly compared with nonsilencing control MSCs and unmanipulated MSCs in the bleomycin model as described earlier. Trichrome staining indicated that MSCs reduced collagen deposition and fibrotic tissue in the lungs (Fig. 6A). HGF knockdown MSCs showed a significantly reduced ability to protect against the development of fibrosis (Fig. 6A); however, nonsilencing control MSCs provided a similar level of protection as unmanipulated MSCs (Fig. 6A). There was a significant difference in the fibrotic score in the lung sections between MSCs and HGF knockdown MSCs (Fig. 6B). This identifies an important role of HGF produced by MSCs in early protection against fibrosis.
MSC-mediated protection of lungs from bleomycin is dependent of HGF production. Lungs were harvested on D28 of the bleomycin study for histological assessment. (A): Representative images of H&E (upper panel) and trichrome (lower panel) stained lung sections (100 ×) show collagen deposition and alveolar damage. Scale bar = 200 μm. (B): Fibrotic score was significantly reduced when MSCs were administered on D0; however, this effect was lost when HGF expression was knocked down in MSCs. n = 5. ∗∗∗, p < .001. Abbreviations: D, day; H&E, hematoxylin and eosin; HGF, hepatocyte growth factor; MSC, mesenchymal stromal cell; Non Sil Ctrl, nonsilencing control; PBS, phosphate-buffered saline; shHGF, shRNA HGF knockdown.
Early MSC Treatment Reduced Lung Epithelial Apoptosis Induced by Bleomycin In Vivo
Bleomycin induces apoptosis in cells through production of reactive oxygen species, inducing DNA strand breaks. In vivo, significant epithelial apoptosis is a predominant feature of bleomycin-induced fibrosis, contributing to the extensive tissue damage observed in this model.
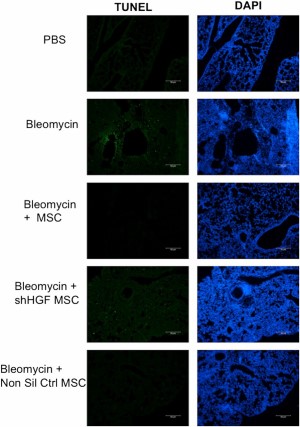
To assess whether this protective ability was due to an antiapoptotic effect as observed in vitro, a terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) assay was performed. Bleomycin exposed lungs displayed high levels of apoptosis in comparison with healthy lungs (Fig. 7). This apoptosis was reduced following MSC administration, indicated by a decreased stain for DNA strand breaks (Fig. 7). HGF has been implicated in prosurvival and antiapoptotic signaling [20–22] in the context of fibrosis and other inflammatory disorders. Therefore, we tested the hypothesis that MSC-derived HGF mediated the antiapoptotic effect observed in early MSC treatment of bleomycin in vivo. HGF knockdown MSCs but not nonsilencing MSCs displayed a decreased ability to inhibit the bleomycin induced apoptosis, supporting its role in MSC prevention of apoptosis (Fig. 7).
MSC-mediated protection of lungs from bleomycin through reduction of apoptosis in an HGF-dependent manner. TUNEL analysis was carried out on lung sections to demonstrate that bleomycin induced apoptosis in the lungs, although MSC treatment reduced this. However, this antiapoptotic effect was lost when mice were treated with HGF KD MSCs, demonstrating that MSCs require HGF to protect against apoptosis. Tissue was counter-stained with DAPI. Representative images from n = 5 mice per group. Scale bars = 50 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; HGF, hepatocyte growth factor; MSC, mesenchymal stromal cell; Non Sil Ctrl, nonsilencing control; PBS, phosphate-buffered saline; shHGF MSC, shRNA HGF knockdown MSC; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling.
Discussion
MSC manipulation of pulmonary cells was examined in vitro to explore potential benefits that might be exploited in an in vivo model of pulmonary fibrosis. The lung is one of the first sites where MSCs can be detected after intravenous administration in mice [31]. Some studies in pulmonary disease models suggest MSCs mediate their effects through engraftment in the lung and differentiation to alveolar cells, resulting in repair of damaged tissue [11, 12]. Conversely, it has been proposed that MSCs mediate repair through cell education and trophic manipulation of the microenvironment [9, 32–34]. Given the heterogeneity of in vitro cultured MSCs, the concepts of regeneration and trophic repair may not be mutually exclusive [33, 35]. In this study, MSC CM was shown to have dynamic effects pertaining to fibrotic therapy, encouraging the migration and proliferation of alveolar epithelial cells while suppressing the activation and proliferation of pulmonary fibroblasts. The conditioned media also displayed antiapoptotic effects in lung epithelial cells, where apoptosis was induced through the addition of the chemotherapeutic drug cisplatin.
Early indications suggested that MSC CM promoted the migration of both fibroblasts and epithelial cells to the site of damage. However, more in-depth analysis confirmed alternate effects with MSC CM promoting the proliferation of alveolar epithelial cells, although fibroblast proliferation was significantly inhibited. These results demonstrate that MSCs favor the migration and proliferation of epithelial cells, and although MSCs encourage the migration of fibroblasts, they also inhibit fibroblast proliferation and activation. Contrary to these findings, a study by Smith et al. [36] showed paracrine signaling by MSCs increased the migration and proliferation of dermal fibroblasts. However, Smith et al. used dermal fibroblast in their study, which may react differently to pulmonary fibroblasts used here: an approach more relevant to pulmonary fibrosis studies.
Taken together, these results support a role for MSCs in pulmonary repair and treatment of fibrosis. Fibroblasts play a vital role in wound healing, migrating to the site of damage where they are involved in wound contraction and closure. Here, MSCs were shown to actively promote fibroblast migration while also controlling the level of proliferation and inhibiting fibroblast activation. Pathogenesis is associated with the disproportionate activation of fibroblasts leading to excessive collagen deposition. This study demonstrates the capacity for MSCs to regulate two major aspects of fibroblast homeostasis allowing for normal wound repair. In line with these findings, others have demonstrated the capacity for MSC CM to control fibroblast activation through downregulating expression of phosphorylated AKT and fibronetin [37]. In addition, MSCs promoted both the migration and proliferation of alveolar epithelial cells, thus consolidating their potential role in tissue repair by encouraging re- epithelialization. Data from others groups also demonstrate the capacity for base line MSC CM to support migration and proliferation of epithelial cell [38, 39]; however, others failed to demonstrate this effect but showed that MSC CM generated in the presence of proinflammatory cytokine stimulation (interferon γ [IFN-γ] and TNF-α) enhanced epithelial cell migration over and above control medium [40]. The MSC CM displayed significant antiapoptotic effects for alveolar epithelial cells mediated in a HGF dependent manner. Apoptosis of epithelial cells in areas of excessive collagen deposition contributes to the impaired wound healing and scarring of lung tissue. Similar findings have demonstrated the capacity for MSC CM to protect airway epithelial cells against apoptosis in vitro but have failed to define the mechanism of action involved [41]. The promotion of epithelial migration and proliferation in conjunction with an antiapoptotic effect in lung epithelial cells highlights the potential of MSCs as a therapy for the prevention of fibrosis mediated through their soluble factors.
Data from a number of studies on fibroblast proliferation suggested a role for PGE2 [27, 42, 43]. In vitro studies demonstrated that alveolar epithelial cell inhibition of fibroblast proliferation was mediated through PGE2 signaling [27]. Here, MSC production of PGE2 was blocked using a COX-2 inhibitor (NS398). CM from NS398-treated MSCs was no longer capable of inhibiting fibroblast proliferation; however, CM from HGF knockdown MSCs maintained capacity to inhibit fibroblast proliferation. These novel data demonstrate the specificity of this effect mediated by PGE2 and not HGF and highlights MSC production of PGE2 as a potential therapeutic candidate in treatment of early fibrosis. To explore MSC potential in repair of established fibrosis, the soluble factor responsible for MSC-induced epithelial expansion was examined. A number of growth factors including keratinocyte growth factor (KGF), epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF) have been implicated in their role in promoting epithelial cell proliferation [44–46].
The presence of KGF in the MSC secretome has been associated with attenuation of Escherichia coli-induced lung injury [47] and in ventilator induced lung injury [48]. A partial role has been identified for VEGF in MSC protection against a cigarette smoke induced lung injury model [49]. However, HGF is known to play an important role in the setting of fibrosis and a number of studies have investigated the functional capacity of HGF in the context of promoting epithelial repair in fibrotic lung disease [15, 50, 51]. An essential role for HGF in MSC-induced proliferation and the antiapoptotic protection of epithelial cells was demonstrated here using conditioned medium from HGF knockdown cells. Similar results were observed in fetal intestinal epithelial cells where HGF secreted by MSCs enhanced their viability and proliferation [52]. Recently, in another model of lung disease, we have demonstrated that MSC CM is less efficacious than MSCs and this is likely associated with the capacity for MSC to continually secrete trophic factors such as HGF (Kennelly et al., manuscript in preparation). Therefore, the capacity for MSCs to protect against fibrotic disease and the mechanism of action involved was investigated in the bleomycin model.
Bleomycin is a resolving model of pathogenesis [53], and although it has drawbacks in that it does not recapitulate all aspects of the human disease, it is the model most often used to test therapeutic efficacy in idiopathic pulmonary fibrosis. The model is now known to divide into two distinct phases: an initial inflammatory phase brought about by immune cell infiltration to the site of damage, followed by a fibrotic phase resulting from fibroblast recruitment and proliferation [54]. The timing of administration of potential therapeutics is essential for distinguishing between preventative anti-inflammatory therapy and antifibrotic therapy. Based on this observation, we chose to examine the therapeutic efficacy of MSC administered early on day 0 or late on day 9 when the fibrotic phase had been initiated.
The in vivo model of fibrosis demonstrated that early administration of allogeneic MSCs protected the lung architecture from bleomycin-induced damage. Early MSC administration significantly reduced fibrosis, apoptosis, and the production of the proinflammatory cytokine IL-1β in the lungs. A study carried out by Lan et al. [37] demonstrated similar outcomes with reduced fibrotic score and decreased IL-1β and IL-6 in a bleomycin model following MSC administration on day 3. The beneficial effects of MSC-derived HGF observed in vitro (in terms of wound healing and apoptosis) were recapitulated in vivo with significant increases in HGF detected in lungs of mice receiving early administration of MSCs. Moreover, other studies have demonstrated elevated levels of HGF and decreased apoptosis in the lung following MSC administration, [37, 55] supporting these findings.
In contrast, delayed MSC dosing failed to protect, resulting in significantly higher pathological scoring, no decrease in IL-1β, and no increase in HGF when compared with the MSC day 0 treatment group. Our findings are in line with other studies [11, 56] demonstrating that protective effects were observed only from MSCs administered directly after bleomycin challenge and not at day 9 after fibrotic disease has been initiated.
Administration of therapeutics at day 0 is common practice in bleomycin animal models and reports have claimed beneficial results from MSCs under these conditions [12, 13, 57]. Early administration of MSCs reduced pathology but not by ameliorating direct fibrosis. Delayed administration had no effect on reducing established fibrosis and was not as effective at reducing inflammation. These results have important implications on future therapeutic candidate studies, with the success of potential antifibrotic therapies being determined by their efficacy following delayed administration in the bleomycin model.
Many studies have shown the capacity for MSCs to protect against bleomycin-induced lung fibrosis [12, 13, 37, 57, 58]. These studies have implicated a range of mechanisms of action including reduction of IL-1 and TNF-α by IL-1 receptor antagonist [13], amelioration of oxidative stress, endoplasmic reticulum stress, and TGF-β1 production in alveolar epithelial cells in a staniocalcin-1 dependent mechanism [58]. Evidence from the literature supports a protective role for HGF in fibrotic lung disease. Apoptotic cells have been shown to modulate bleomycin lung fibrosis through the production of HGF alone [59] or in combination with PGE2 [60]. Notably, a recent study has identified the role for a secreted factor in MSC protection against lung fibrosis and acute lung injury using conditioned medium from amniotic membrane derived cells [61] or proinflammatory cytokine-activated MSCs [62]. Similar observations have been made in other cells types including induced pluripotent stem cells, with reduced lung fibrosis mediated in part by HGF [63]. In line with this, hypoxia preconditioning or overexpression of HGF in MSCs leads to better efficacy of MSCs in preventing lung fibrosis and this was associated with increased levels of HGF [37, 64]. Given the partial role identified for HGF in MSC protection against bleomycin-induced lung fibrosis, it is plausible that other secreted factors such as PGE2 might play a complementary role.
Conclusion
Our study is the first study to definitively show the importance of (naturally derived) HGF in MSC protection in the bleomycin model in providing clear mechanistic studies translating in vitro finding to a preclinical model of bleomycin-induced fibrosis. Moreover, the data herein support the theory that MSCs have cytoprotective properties and highlight the important role of MSCs in the repair process through prevention of apoptosis, suppression of fibroblast proliferation, and promotion of epithelial cell proliferation. In vivo, the protective effect of MSCs may have far-reaching clinical applications but will depend on the accuracy of timing in administration.
Acknowledgments
We wish to express our thanks to Marc Healy, Laura Cahill, and Jennifer Corbett for their assistance with the in vivo models. This work was supported by grants from Science Foundation Ireland under Grant 09/SRC/B1794. H.K. was supported through a BioAnalysis and Therapeutics Ph.D. scholarship funded by the Higher Education Authority under Cycle 5 of the Programme for Research in Third-Level Institutions. K.E. was supported by a Health Research Board Translational Medicine postdoctoral research fellowship and a Marie Curie Career Integration Grant.
Author Contributions
E.F.C., H.K., and F.C.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript; B.P.M.: conception and design, financial support, data analysis and interpretation, final approval of the manuscript; K.E.: conception and design, data analysis and interpretation, manuscript writing, financial support, final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
Author notes
Contributed equally.