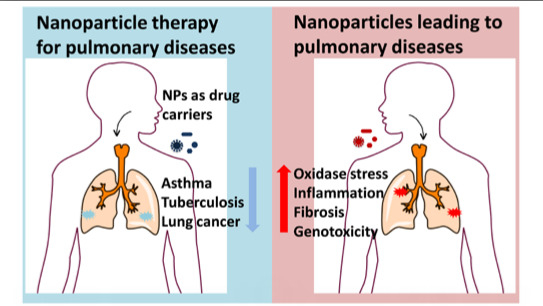
Right or Left: The Role of Nanoparticles in Pulmonary Diseases
Abstract
:1. Introduction
2. Nanoparticle Therapy in Lung Diseases
| Nanoparticle | Animal Models | Exposure Method | Description | Use | Ref. |
|---|---|---|---|---|---|
| poly(l-aspartic acid-co-lactic acid)/DPPE co-polymer NPs | Mouse xenograft model | intraperitoneal injection | amphiphilic biodegradable poly(l-aspartic acid-co-lactic acid)/DPPE co-polymer NPs loaded with doxorubicin (DOX) | Lung cancer | [3] |
| PEG-dendritic block telodendrimer | OVA-exposed mice | intravenous injection | self-assembling nanoparticles containing Dex | Allergic Asthma | [11] |
| pDNA nanoparticles (NPs) | OVA-exposed mice | intranasal | chitosan/IFN-gamma pDNA NPs (CIN) | Allergic Asthma | [12] |
| poly (dl-lactideco-glycolide) NPs | M. tuberculosis infected guinea pigs | inhalation | poly (dl-lactideco-glycolide) loaded with ATDs | Tuberculosis | [13] |
| polybutyl cyanoacrylate NPs | Mouse xenograft model | intravenous injection | DOX-loaded NPs were incorporated into inhalable effervescent and non-effervescent carrier particles using a spray-freeze drying technique | Lung cancer | [14] |
| poly(beta-amino ester) (PBAE) polymers | Mouse xenograft model | intratumoral injection | biodegradable PBAE polymers that self-assemble with DNA | Lung cancer | [15] |
| LPH (liposome-polycation-hyaluronic acid) nanoparticles | Mouse xenograft model | intravenous injection | LPH nanoparticle formulation modified with tumor-targeting single-chain antibody fragment for systemic delivery of siRNA and microRNA efficiently downregulated the target genes (c-Myc/MDM2/VEGF) | Cancer lung metastasis | [16] |
2.1. Asthma
2.2. Tuberculosis
2.3. Lung Cancer

3. The Pulmonary Diseases Caused by Nanoparticle Exposure
3.1. Deposition and Clearance of Nanoparticle in the Lung
3.2. Pulmonary Diseases after Nanoparticle Exposure
3.2.1. Asthma
3.2.2. Granuloma

3.2.3. Lung Cancer
3.3. Pathobiological Processes Caused by Nanoparticles in the Lung

3.3.1. Oxidative Stress
3.3.2. Inflammation
3.3.3. Genotoxicity
3.3.4. Fibrosis
| Nanoparticles | In Vivo Exposure Procedure (Dose, Period, Animal Model) | Physicochemical Properties | Lung Injury and Lung Disease | Ref. |
|---|---|---|---|---|
| MWCNT | intratracheal instillation once a day for two consecutive days; 0.6 mg/rat; 30 days; SH rat | Length | Long MWCNTs (20–50 μm) but not short MWCNTs (0.5–2 μm) exhibit increased fibroblast proliferation, collagen deposition and granuloma formation in lung tissue. | [33] |
| instillation 100 µg/mice; 1, 7, 30, 90, o r 180 days; Male Balb/c mice | Suface modification NT1: none NT2: carboxylic polyacid polymer NT3: polystyrene polybutadiene polymethylacrylate(PMMA) Surface area NT1: 227. 54 m2/g NT2: 54.1 m2/g NT3: 34 m2/g | NT1 and NT2, not NT3, induced inflammatory response and these effects were observed 24 h post-instillation and lasted up to 1 month. | [55] | |
| intratracheal instillation(single); 2 mg/rat; 3 days; female wistar rats | Thickness (diameter) MWCNT9.4: 9.4 ± 0.3 nm MWCNT70: 70 ± 2 nm | Thin MWCNTs induced an inflammatory lung response when instilled in rats. Conversely, thick MWCNTs appeared to be of low toxicity. | [59] | |
| Graphene | intratracheal instillation (single); 50 μg/mouse; 21 days; C57BL/6 mice | Surface modification (covalent oxidation) aggregation | GO increased the rate of mitochondrial respiration and the generation of ROS, activating inflammation. | [41] |
| Nickel nanowires | Pharyngeal aspiration; 7 days; 50 mg/mice; female C57BL/6 mice | Length Long: 24 ± 7 µmShort: 4.3 ± 1 µm | Long nanowires led to a moderate inflammatory response and a strong granulomatous response in the peripheral airways, but short ones did not cause these responses. | [60] |
| Nano-TiO2 | nose-only exposure for 6 h; 20 mg/m3; 16 h; Rats | Agglomeration state: Large agglomerate (LA): >100 nm Small agglomerate (SA): <100 nm Size: 5 nm 10–30 nm 50 nm | 5 nm SA particles caused a noted increase in cytotoxic effects, while oxidative damage was less compared to 10–30 and 50 nm SA particles. In SA and LA aerosols, the 10–30 nm TiO2 NP induced the most marked pro-inflammatory effects. | [61] |
| Intratracheal instillation(single); 1 or 5 mg/kg; 24 h, 1 week, 1 month, and 3 months; Male rats | Surface area: (1) Nanoscale rods Dlong = 92–233 nm Dwide = 20–35 nm 26.5 m2/g (2) Nanoscale dots: 5.8–6.1 nm spherical 169.4 m2/g | No significant difference in pulmonary inflammation for long-term exposure. | [62] |
4. Secondary Diseases after Pulmonary Diseases Caused by Nanoparticle Exposure
4.1. Cardiovascular Disease
4.2. Diseases in Other Tissues

5. Conclusions

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miao, J.; Miyauchi, M.; Simmons, T.J.; Dordick, J.S.; Linhardt, R.J. Electrospinning of nanomaterials and applications in electronic components and devices. J. Nanosci. Nanotechnol. 2010, 10, 5507–5519. [Google Scholar]
- Anik, U.; Cubukcu, M.; Yavuz, Y. Nanomaterial-based composite biosensor for glucose detection in alcoholic beverages. Artif. Cells Nanomed. Biotechnol. 2013, 41, 8–12. [Google Scholar] [PubMed]
- Han, S.; Liu, Y.; Nie, X.; Xu, Q.; Jiao, F.; Li, W.; Zhao, Y.; Wu, Y.; Chen, C. Efficient delivery of antitumor drug to the nuclei of tumor cells by amphiphilic biodegradable poly(l-aspartic acid-co-lactic acid)/DPPE co-polymer nanoparticles. Small 2012, 8, 1596–1606. [Google Scholar] [PubMed]
- Xu, L.; Liu, Y.; Chen, Z.; Li, W.; Wang, L.; Wu, X.; Ji, Y.; Zhao, Y.; Ma, L.; Shao, Y.; et al. Surface-engineered gold nanorods: Promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012, 12, 2003–2012. [Google Scholar] [PubMed]
- Morganti, P. Use and potential of nanotechnology in cosmetic dermatology. Clin. Cosmet. Investig. Dermatol. 2010, 3, 5–13. [Google Scholar] [PubMed]
- Peters, A.; Wichmann, H.E.; Tuch, T.; Heinrich, J.; Heyder, J. Respiratory effects are associated with the number of ultrafine particles. Am. J. Respir. Crit. Care Med. 1997, 155, 1376–1383. [Google Scholar] [PubMed]
- Pope, C.A., 3rd; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [PubMed]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [PubMed]
- Jud, C.; Clift, M.J.; Petri-Fink, A.; Rothen-Rutishauser, B. Nanomaterials and the human lung: What is known and what must be deciphered to realise their potential advantages? Swiss Med. Wkly. 2013, 143, w13758. [Google Scholar]
- Donaldson, K.; Poland, C.A. Inhaled nanoparticles and lung cancer—What we can learn from conventional particle toxicology. Swiss Med. Wkly. 2012, 142, w13547. [Google Scholar]
- Kenyon, N.J.; Bratt, J.M.; Lee, J.; Luo, J.; Franzi, L.M.; Zeki, A.A.; Lam, K.S. Self-assembling nanoparticles containing dexamethasone as a novel therapy in allergic airways inflammation. PLoS One 2013, 8, e77730. [Google Scholar] [PubMed]
- Kumar, M.; Kong, X.; Behera, A.K.; Hellermann, G.R.; Lockey, R.F.; Mohapatra, S.S. Chitosan IFN-gamma-pDNA Nanoparticle (CIN) Therapy for Allergic Asthma. Genet. Vaccines Ther. 2003, 1, 3. [Google Scholar] [PubMed]
- Pandey, R.; Sharma, A.; Zahoor, A.; Sharma, S.; Khuller, G.K.; Prasad, B. Poly (dl-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J. Antimicrob. Chemother. 2003, 52, 981–986. [Google Scholar] [PubMed]
- Roa, W.H.; Azarmi, S.; Al-Hallak, M.H.; Finlay, W.H.; Magliocco, A.M.; Lobenberg, R. Inhalable nanoparticles, a non-invasive approach to treat lung cancer in a mouse model. J. Control. Release 2011, 150, 49–55. [Google Scholar] [PubMed]
- Kamat, C.D.; Shmueli, R.B.; Connis, N.; Rudin, C.M.; Green, J.J.; Hann, C.L. Poly(beta-amino ester) nanoparticle delivery of TP53 has activity against small cell lung cancer in vitro and in vivo. Mol. Cancer Ther. 2013, 12, 405–415. [Google Scholar] [PubMed]
- Chen, Y.; Zhu, X.; Zhang, X.; Liu, B.; Huang, L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol. Ther. 2010, 18, 1650–1656. [Google Scholar] [PubMed]
- Kroegel, C. Global Initiative for Asthma (GINA) guidelines: 15 Years of application. Expert Rev. Clin. Immunol. 2009, 5, 239–249. [Google Scholar] [PubMed]
- Dahl, R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respiratory Med. 2006, 100, 1307–1317. [Google Scholar]
- Fauci, A.S. Infectious diseases: Considerations for the 21st century. Clin. Infect. Dis. 2001, 32, 675–685. [Google Scholar] [PubMed]
- Jemal, A.; Thun, M.J.; Ries, L.A.; Howe, H.L.; Weir, H.K.; Center, M.M.; Ward, E.; Wu, X.C.; Eheman, C.; Anderson, R.U.A.; et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J. Natl. Cancer Inst. 2008, 100, 1672–1694. [Google Scholar] [PubMed]
- Kim, S.C.; Kim, D.W.; Shim, Y.H.; Bang, J.S.; Oh, H.S.; Wan Kim, S.; Seo, M.H. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J. Control. Release 2001, 72, 191–202. [Google Scholar] [PubMed]
- Hitzman, C.J.; Wattenberg, L.W.; Wiedmann, T.S. Pharmacokinetics of 5-fluorouracil in the hamster following inhalation delivery of lipid-coated nanoparticles. J. Pharm. Sci. 2006, 95, 1196–1211. [Google Scholar] [PubMed]
- Da Silva, A.L.; Santos, R.S.; Xisto, D.G.; Alonso Sdel, V.; Morales, M.M.; Rocco, P.R. Nanoparticle-based therapy for respiratory diseases. An. Acad. Bras. Cienc. 2013, 85, 137–146. [Google Scholar]
- Li, Y.F.; Chen, C. Fate and toxicity of metallic and metal-containing nanoparticles for biomedical applications. Small 2011, 7, 2965–2980. [Google Scholar] [PubMed]
- Hoet, P.H.; Bruske-Hohlfeld, I.; Salata, O.V. Nanoparticles—Known and unknown health risks. J. Nanobiotechnol. 2004, 2, 12. [Google Scholar]
- Aalapati, S.; Ganapathy, S.; Manapuram, S.; Anumolu, G.; Prakya, B.M. Toxicity and bio-accumulation of inhaled cerium oxide nanoparticles in CD1 mice. Nanotoxicology 2014, 8, 786–798. [Google Scholar] [PubMed]
- Moolgavkar, S.H.; Brown, R.C.; Turim, J. Biopersistence, fiber length, and cancer risk assessment for inhaled fibers. Inhal. Toxicol. 2001, 13, 755–772. [Google Scholar] [PubMed]
- Oberdorster, G.; Ferin, J.; Lehnert, B.E. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 1994, 102 (Suppl. S5), 173–179. [Google Scholar] [PubMed]
- Han, B.; Guo, J.; Abrahaley, T.; Qin, L.; Wang, L.; Zheng, Y.; Li, B.; Liu, D.; Yao, H.; Yang, J.; et al. Adverse effect of nano-silicon dioxide on lung function of rats with or without ovalbumin immunization. PLoS One 2011, 6, e17236. [Google Scholar] [PubMed]
- Chen, E.Y.; Garnica, M.; Wang, Y.C.; Chen, C.S.; Chin, W.C. Mucin secretion induced by titanium dioxide nanoparticles. PLoS One 2011, 6, e16198. [Google Scholar] [PubMed]
- Song, Y.; Li, X.; Du, X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur. Respir. J. 2009, 34, 559–567. [Google Scholar] [PubMed]
- Hsieh, W.Y.; Chou, C.C.; Ho, C.C.; Yu, S.L.; Chen, H.Y.; Chou, H.Y.; Chen, J.J.; Chen, H.W.; Yang, P.C. Single-walled carbon nanotubes induce airway hyperreactivity and parenchymal injury in mice. Am. J. Respir. Cell Mol. Biol. 2012, 46, 257–267. [Google Scholar] [PubMed]
- Wang, P.; Nie, X.; Wang, Y.; Li, Y.; Ge, C.; Zhang, L.; Wang, L.; Bai, R.; Chen, Z.; Zhao, Y.; et al. Multiwall carbon nanotubes mediate macrophage activation and promote pulmonary fibrosis through TGF-beta/Smad signaling pathway. Small 2013, 9, 3799–3811. [Google Scholar]
- Park, E.J.; Yoon, J.; Choi, K.; Yi, J.; Park, K. Induction of chronic inflammation in mice treated with titanium dioxide nanoparticles by intratracheal instillation. Toxicology 2009, 260, 37–46. [Google Scholar] [PubMed]
- Shvedova, A.A.; Tkach, A.V.; Kisin, E.R.; Khaliullin, T.; Stanley, S.; Gutkin, D.W.; Star, A.; Chen, Y.; Shurin, G.V.; Kagan, V.E.; et al. Carbon nanotubes enhance metastatic growth of lung carcinoma via up-regulation of myeloid-derived suppressor cells. Small 2013, 9, 1691–1695. [Google Scholar] [PubMed]
- Nagai, H.; Okazaki, Y.; Chew, S.H.; Misawa, N.; Yamashita, Y.; Akatsuka, S.; Ishihara, T.; Yamashita, K.; Yoshikawa, Y.; Yasui, H.; et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, E1330–E1338. [Google Scholar] [PubMed]
- Sakamoto, Y.; Nakae, D.; Fukumori, N.; Tayama, K.; Maekawa, A.; Imai, K.; Hirose, A.; Nishimura, T.; Ohashi, N.; Ogata, A. Induction of mesothelioma by a single intrascrotal administration of multi-wall carbon nanotube in intact male Fischer 344 rats. J. Toxicol. Sci. 2009, 34, 65–76. [Google Scholar] [PubMed]
- Sun, Q.; Tan, D.; Ze, Y.; Sang, X.; Liu, X.; Gui, S.; Cheng, Z.; Cheng, J.; Hu, R.; Gao, G.; et al. Pulmotoxicological effects caused by long-term titanium dioxide nanoparticles exposure in mice. J. Hazard. Mater. 2012, 235–236, 47–53. [Google Scholar] [PubMed]
- Ge, C.; Du, J.; Zhao, L.; Wang, L.; Liu, Y.; Li, D.; Yang, Y.; Zhou, R.; Zhao, Y.; Chai, Z.; et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl. Acad. Sci. USA 2011, 108, 16968–16973. [Google Scholar] [PubMed]
- Kim, J.S.; Sung, J.H.; Song, K.S.; Lee, J.H.; Kim, S.M.; Lee, G.H.; Ahn, K.H.; Lee, J.S.; Shin, J.H.; Park, J.D.; et al. Persistent DNA damage measured by comet assay of Sprague Dawley rat lung cells after five days of inhalation exposure and 1 month post-exposure to dispersed multi-wall carbon nanotubes (MWCNTs) generated by new MWCNT aerosol generation system. Toxicol. Sci. 2012, 128, 439–448. [Google Scholar] [PubMed]
- Duch, M.C.; Budinger, G.R.; Liang, Y.T.; Soberanes, S.; Urich, D.; Chiarella, S.E.; Campochiaro, L.A.; Gonzalez, A.; Chandel, N.S.; Hersam, M.C.; et al. Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung. Nano Lett. 2011, 11, 5201–5207. [Google Scholar]
- Xia, T.; Kovochich, M.; Liong, M.; Zink, J.I.; Nel, A.E. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano 2008, 2, 85–96. [Google Scholar] [PubMed]
- Park, E.J.; Yi, J.; Chung, K.H.; Ryu, D.Y.; Choi, J.; Park, K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol. Lett. 2008, 180, 222–229. [Google Scholar] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [PubMed]
- Lee, H.M.; Shin, D.M.; Song, H.M.; Yuk, J.M.; Lee, Z.W.; Lee, S.H.; Hwang, S.M.; Kim, J.M.; Lee, C.S.; Jo, E.K. Nanoparticles up-regulate tumor necrosis factor-alpha and CXCL8 via reactive oxygen species and mitogen-activated protein kinase activation. Toxicol. Appl. Pharmacol. 2009, 238, 160–169. [Google Scholar]
- Li, R.; Ning, Z.; Majumdar, R.; Cui, J.; Takabe, W.; Jen, N.; Sioutas, C.; Hsiai, T. Ultrafine particles from diesel vehicle emissions at different driving cycles induce differential vascular pro-inflammatory responses: Implication of chemical components and NF-kappaB signaling. Part. Fibre Toxicol. 2010, 7, 6. [Google Scholar] [PubMed]
- Mroz, R.M.; Schins, R.P.; Li, H.; Drost, E.M.; Macnee, W.; Donaldson, K. Nanoparticle carbon black driven DNA damage induces growth arrest and AP-1 and NFkappaB DNA binding in lung epithelial A549 cell line. J. Physiol. Pharmacol. 2007, 58 (Suppl. S5), 461–470. [Google Scholar] [PubMed]
- Hubbs, A.F.; Mercer, R.R.; Benkovic, S.A.; Harkema, J.; Sriram, K.; Schwegler-Berry, D.; Goravanahally, M.P.; Nurkiewicz, T.R.; Castranova, V.; Sargent, L.M. Nanotoxicology—A pathologist’s perspective. Toxicol. Pathol. 2011, 39, 301–324. [Google Scholar] [PubMed]
- Li, S.; He, P.; Dong, J.; Guo, Z.; Dai, L. DNA-directed self-assembling of carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 14–15. [Google Scholar] [PubMed]
- Gillespie, P.A.; Kang, G.S.; Elder, A.; Gelein, R.; Chen, L.; Moreira, A.L.; Koberstein, J.; Tchou-Wong, K.M.; Gordon, T.; Chen, L.C. Pulmonary response after exposure to inhaled nickel hydroxide nanoparticles: Short and long-term studies in mice. Nanotoxicology 2010, 4, 106–119. [Google Scholar] [PubMed]
- Cho, W.S.; Duffin, R.; Poland, C.A.; Duschl, A.; Oostingh, G.J.; Macnee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology 2012, 6, 22–35. [Google Scholar] [PubMed]
- Shvedova, A.A.; Kisin, E.; Murray, A.R.; Johnson, V.J.; Gorelik, O.; Arepalli, S.; Hubbs, A.F.; Mercer, R.R.; Keohavong, P.; Sussman, N.; et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: Inflammation, fibrosis, oxidative stress, and mutagenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L552–L565. [Google Scholar] [PubMed]
- Crouzier, D.; Follot, S.; Gentilhomme, E.; Flahaut, E.; Arnaud, R.; Dabouis, V.; Castellarin, C.; Debouzy, J.C. Carbon nanotubes induce inflammation but decrease the production of reactive oxygen species in lung. Toxicology 2010, 272, 39–45. [Google Scholar] [PubMed]
- Schulz, M.; Ma-Hock, L.; Brill, S.; Strauss, V.; Treumann, S.; Groters, S.; Ravenzwaay, B.; Landsiedel, R. Investigation on the genotoxicity of different sizes of gold nanoparticles administered to the lungs of rats. Mutat. Res. 2012, 745, 51–57. [Google Scholar] [PubMed]
- Tabet, L.; Bussy, C.; Setyan, A.; Simon-Deckers, A.; Rossi, M.J.; Boczkowsk, J.; Lanone, S. Coating carbon nanotubes with a polystyrene-based polymer protects against pulmonary toxicity. Part. Fibre Toxicol. 2011, 8, 3. [Google Scholar] [PubMed]
- Osier, M.; Oberdorster, G. Intratracheal inhalation vs. intratracheal instillation: Differences in particle effects. Fundam. Appl. Toxicol. 1997, 40, 220–227. [Google Scholar] [PubMed]
- Warheit, D.B.; Brock, W.J.; Lee, K.P.; Webb, T.R.; Reed, K.L. Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: Impact of surface treatments on particle toxicity. Toxicol. Sci. 2005, 88, 514–524. [Google Scholar] [PubMed]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [PubMed]
- Fenoglio, I.; Aldieri, E.; Gazzano, E.; Cesano, F.; Colonna, M.; Scarano, D.; Mazzucco, G.; Attanasio, A.; Yakoub, Y.; Lison, D.; et al. Thickness of multiwalled carbon nanotubes affects their lung toxicity. Chem. Res. Toxicol. 2012, 25, 74–82. [Google Scholar]
- Poland, C.A.; Byrne, F.; Cho, W.S.; Prina-Mello, A.; Murphy, F.A.; Davies, G.L.; Coey, J.M.; Gounko, Y.; Duffin, R.; Volkov, Y.; et al. Length-dependent pathogenic effects of nickel nanowires in the lungs and the peritoneal cavity. Nanotoxicology 2012, 6, 899–911. [Google Scholar] [PubMed]
- Noël, A.; Maghni, K.; Cloutier, Y.; Dion, C.; Wilkinson, K.J.; Hallé, S.; Tardif, R.; Truchon, G. Effects of inhaled nano-TiO2 aerosols showing two distinct agglomeration states on rat lungs. Toxicol. Lett. 2012, 214, 109–119. [Google Scholar] [PubMed]
- Warheit, D.B.; Webb, T.R.; Sayes, C.M.; Colvin, V.L.; Reed, K.L. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: Toxicity is not dependent upon particle size and surface area. Toxicol. Sci. 2006, 91, 227–236. [Google Scholar] [PubMed]
- Nounou, H.; Attia, H.; Shalaby, M.; Arafah, M. Oral exposure to zinc oxide nanoparticles induced oxidative damage, inflammation and genotoxicity in rat’s lung. Life Sci. J. 2013, 10, 1. [Google Scholar]
- Pope, C.A., 3rd; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [PubMed]
- Kang, G.S.; Gillespie, P.A.; Gunnison, A.; Moreira, A.L.; Tchou-Wong, K.M.; Chen, L.C. Long-term inhalation exposure to nickel nanoparticles exacerbated atherosclerosis in a susceptible mouse model. Environ. Health Perspect. 2011, 119, 176–181. [Google Scholar] [PubMed]
- Yamawaki, H.; Iwai, N. Mechanisms underlying nano-sized air-pollution-mediated progression of atherosclerosis: Carbon black causes cytotoxic injury/inflammation and inhibits cell growth in vascular endothelial cells. Circ. J. 2006, 70, 129–140. [Google Scholar] [PubMed]
- Erdely, A.; Hulderman, T.; Salmen, R.; Liston, A.; Zeidler-Erdely, P.C.; Schwegler-Berry, D.; Castranova, V.; Koyama, S.; Kim, Y.A.; Endo, M.; et al. Cross-talk between lung and systemic circulation during carbon nanotube respiratory exposure. Potential biomarkers. Nano Lett. 2009, 9, 36–43. [Google Scholar] [PubMed]
- Blum, J.L.; Xiong, J.Q.; Hoffman, C.; Zelikoff, J.T. Cadmium associated with inhaled cadmium oxide nanoparticles impacts fetal and neonatal development and growth. Toxicol. Sci. 2012, 126, 478–486. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Zhu, T.; Chen, C.; Liu, Y. Right or Left: The Role of Nanoparticles in Pulmonary Diseases. Int. J. Mol. Sci. 2014, 15, 17577-17600. https://doi.org/10.3390/ijms151017577
Lu X, Zhu T, Chen C, Liu Y. Right or Left: The Role of Nanoparticles in Pulmonary Diseases. International Journal of Molecular Sciences. 2014; 15(10):17577-17600. https://doi.org/10.3390/ijms151017577
Chicago/Turabian StyleLu, Xuefei, Tao Zhu, Chunying Chen, and Ying Liu. 2014. "Right or Left: The Role of Nanoparticles in Pulmonary Diseases" International Journal of Molecular Sciences 15, no. 10: 17577-17600. https://doi.org/10.3390/ijms151017577