- 1Department of Immunology and Allergy, Sydney Children’s Hospital Randwick, Sydney, NSW, Australia
- 2School of Women and Children’s Health, University of New South Wales, Sydney, NSW, Australia
- 3Inflammation Division, The Walter and Eliza Hall Institute of Medical Research, Parkville, VIC, Australia
- 4Department of Medical Biology, The University of Melbourne, Parkville, VIC, Australia
- 5Children’s Hospital at Westmead, Westmead, NSW, Australia
- 6Department of Medical Genetics, Sydney Children’s Hospital, Randwick, NSW, Australia
- 7Department of Anatomical Pathology, NSW Health Pathology, Prince of Wales Hospital, Sydney, NSW, Australia
- 8School of Medical Sciences, University of New South Wales, Sydney, NSW, Australia
- 9Clinical Immunogenomics Consortium Australia, Sydney, NSW, Australia
- 10Australian Autoinflammatory Diseases Registry, Melbourne, VIC, Australia
Background: Mutations in the gene encoding stimulator of interferon genes (STING) underlie a type I interferon (IFN) associated disease, STING-associated vasculopathy with onset in infancy (SAVI). Patients suffer cutaneous vasculopathy and interstitial lung disease, but are not known to suffer life-threatening infection.
Case: We describe a child who presented with Pneumocystis jirovecii pneumonia in early life, from which he recovered. He went on to suffer failure to thrive, developmental delay, livedo reticularis, and vesicular rash, but without cutaneous vasculitis, and with normal C-reactive protein and erythrocyte sedimentation rates. At 3 years of age, he developed life-threatening pulmonary hypertension.
Methods: Whole genome sequencing (WGS) was performed using the Illumina HiSeqX10 platform and the Seave platform was used for bioinformatic analysis. mRNA expression of IFN-stimulated genes and inflammatory cytokines from peripheral blood mononuclear cells was determined by quantitative polymerase chain reaction. Luciferase assay was used to model IFNβ and NF-κB activity in vitro.
Results: WGS revealed a de novo mutation p.Arg284Ser in STING at an amino acid previously associated with SAVI. Although this mutation did not fall in the dimerization domain (DD), mRNA analysis revealed constitutive IFN-gene activation consistent with an interferonopathy, which correlated to STING activation in vitro. The patient was treated with corticosteroids and the JAK inhibitor Ruxolitinib, resulting in a rapid improvement of pulmonary hypertension, general well-being, and resolution of the IFN gene signature. However, he did go on to evolve a nasal septal erosion suggesting incomplete control of disease.
Conclusion: This case provides molecular evidence to support the p.Arg284Ser variant in STING exerting pathogenicity through a gain-of-function mechanism. The lack of cutaneous vasculitis or elevated systemic inflammatory markers, and the occurrence of an opportunistic infection are notable, and raise the possibility that variants outside the STING DD may potentially manifest with an atypical SAVI phenotype. Nevertheless, there was an objective clinical improvement in response to JAK inhibition.
Introduction
Type I interferons (IFNs α and β) play a crucial role in immune mediated protection from viral infection. Stimulator of interferon genes (STING), encoded by the gene TMEM173, is a key intermediary in activating this type I IFN response (1). Once activated, STING induces the phosphorylation of transcription factors IRF3 and NF-κB, promoting their nuclear translocation and the transcription of IFNs and inflammatory cytokines, respectively. Type I IFNs act via the IFN α/β receptor (IFNAR), which in turn activates a number of down-stream signaling pathways, most notably the JAK–STAT pathway. IFNAR agonism leads to the transcriptional regulation of over 2,000 genes, referred to as IFN-stimulated genes (ISGs). Heterozygous gain-of-function (GoF) mutations in TMEM173 result in constitutive activation of STING and the clinical syndrome known as STING-associated vasculopathy with onset in infancy (SAVI) (2). To date, a total of seven GoF mutations have been reported to induce SAVI, a disease characterized by neonatal–onset inflammation with raised inflammatory markers, cutaneous vasculopathy, and interstitial lung disease (2–9). SAVI has been classified an as interferonopathy as it is characterized by elevated levels of type I IFNs and ISGs. Activation of STING also triggers NF-κB, and mouse models suggest that this could contribute to the pathogenesis of SAVI (10). Typically, activation of NF-κB is associated with increased C reactive protein (CRP) in patients with inflammation, and CRP is also elevated in SAVI.
Case
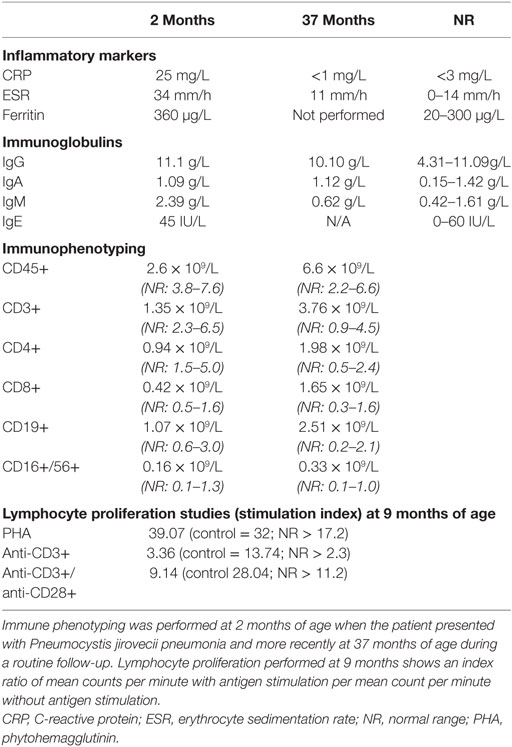
A 3-year-old boy born to non-consanguineous parents of Chinese/Malaysian ethnicity presented at 2 months of age with acute respiratory distress requiring mechanical ventilation and an interstitial pneumonitis on X-ray (Figure 1A). Bronchoalveolar lavage identified Pneumocystis jirovecii by both PCR and immunofluorescence. He was treated with co-trimoxazole and made a full recovery (Figure 1B). Immunological work up performed at the time revealed normal immunoglobulin levels, with CD4+ and CD8+ lymphopenia (Table 1). The CD8+ count normalized rapidly but his CD4+ lymphopenia persisted until he was 5 months of age. CD19+ B cell and NK cell numbers were normal; however, the B cell numbers climbed and have remained high (range 2.5–6.0 × 109/L—reference range 0.2–2.1 × 109/L). T cell immunophenotyping identified no abnormality (Table 1). Mitogen-specific T-cell blastogenesis with phytohemagglutinin (PHA) was preserved; however, T-cell stimulation with anti-CD3 in early life was absent, and stimulation with anti-CD3/anti-CD28 at 9 months of age was reduced threefold compared with a control sample (Table 1). He displayed features of global developmental delay at 7 months and was noted to be hypertonic floppy with normal deep tendon reflexes and a normal MRI of the brain. These features improved slowly and required intensive allied health (physiotherapy, occupational therapy, and dietician) support that is ongoing. He had failure to thrive complicated by frequent vomiting necessitating insertion of a gastrostomy at 10 months of age. Endoscopy revealed a thickened (but non-occlusive) pyloric antrum. He developed acral erythematous papules and vesicles on his upper and lower limbs. Skin biopsy revealed superficial subcorneal neutrophil microabscess formation with superficial and deep dermal and subcutaneous neutrophilia (Figure 1C). Neutrophils were also present within the lumen of a sweat duct. There was no evidence of vasculitis, although interstitial leukocytoclasis was present. The acral papules (Figure 1D) gradually resolved around 9 months while the skin of his extremities demonstrated a livedo racemosa pattern (Figure 1E). Notably, at the time of presentation he had raised inflammatory markers; however, these decreased to within normal range when tested during a routine follow-up (CRP < 3 mg/L, erythrocyte sedimentation rate < 14 mm/h) (Table 1).
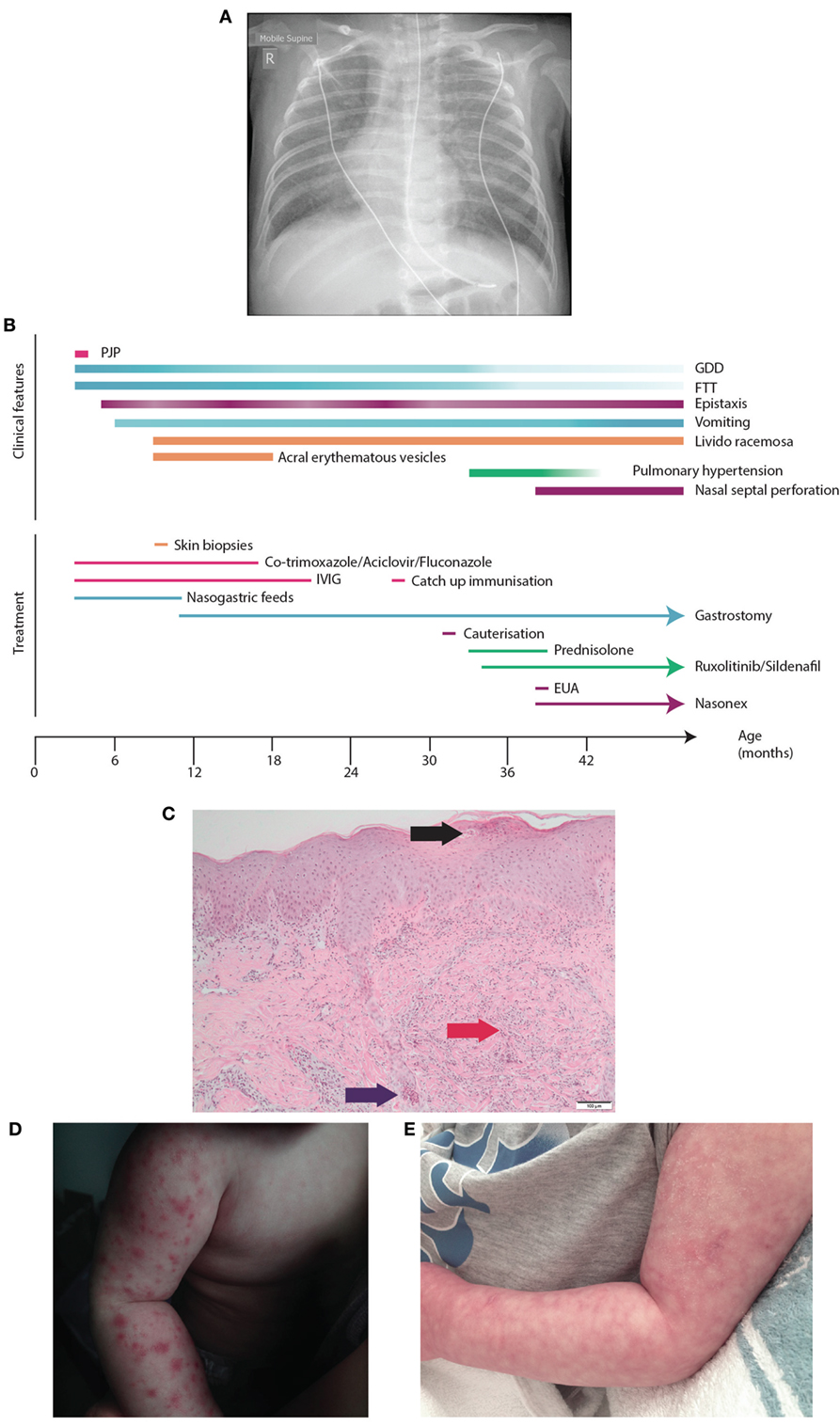
Figure 1. Presentation of SAVI R284S. (A) Chest X-ray of patient demonstrating diffuse hazy changes present bilaterally in the lung fields. The X-ray appearance was consistent with an interstitial pneumonitis which was initially attributed to his Pneumocystis jirovecci infection requiring non-invasive ventilator support and then an escalation to intubation and ventilator support. (B) Time-line for disease manifestation and treatments undergone by the index case. (C) Histopathology of the vesicles from the skin biopsy of the arm demonstrating a pustule (black arrow) with dermal neutrophilia (red arrow) and associated leukocytoclasis. Neutrophils are also present within the lumen of a sweat duct (blue arrow). (D) Photograph of the acral erythromatous papules and vesicles that were present on both upper and lower limbs which resolved into (E) a livedo racemosa pattern.
The patient was managed as a primary immunodeficiency with prophylactic co-trimoxazole and immunoglobulin replacement. He did not suffer from any further significant infections. Co-trimoxazole was ceased at 2 years of age and immunoglobulin replacement ceased at 21 months of age, since when he has remained free of infections. The patient underwent routine immunization and demonstrated adequate post vaccination serological responses.
At 33 months, the patient developed a pulmonary hypertensive crisis episode characterized by hypoxia following minor stimulation. Electrocardiogram suggested right ventricular (RV) hypertrophy, subsequently confirmed by echocardiogram which demonstrated suprasystemic pulmonary hypertension and a pressure loaded RV. Notably cardiac echocardiogram performed at age 21 months was normal. High resolution computed tomography of the chest to identify a pulmonary cause was abandoned due to hypertensive crises on stimulation. He was subsequently started on the pulmonary specific vasodilator, sildenafil.
To establish if there was a genetic cause of this disease, whole genome sequencing (WGS) was performed on the family trio. This identified a novel de novo missense variant c.852G>T in exon 7 of 8 in the gene TMEM173, encoding p.Arg284Ser in the protein STING (Figure 2A). The variant was confirmed by Sanger sequencing, and is not present in the Exac database (11). In silico analysis predicted the mutation to be pathogenic based on; the physiochemical difference between arginine and serine (Grantham score 110); SIFT prediction (deleterious), PolyPhen (probably damaging), CADD (scaled = 26.7), mutation taster (disease causing), and Provean (deleterious). Interestingly, a previous case report documented a different de novo change in the same amino acid residue; c850A>G (pArg284Gly, R284G) in a patient suffering from SAVI with upregulation of IFN genes secondary to constitutive STING activation (8).
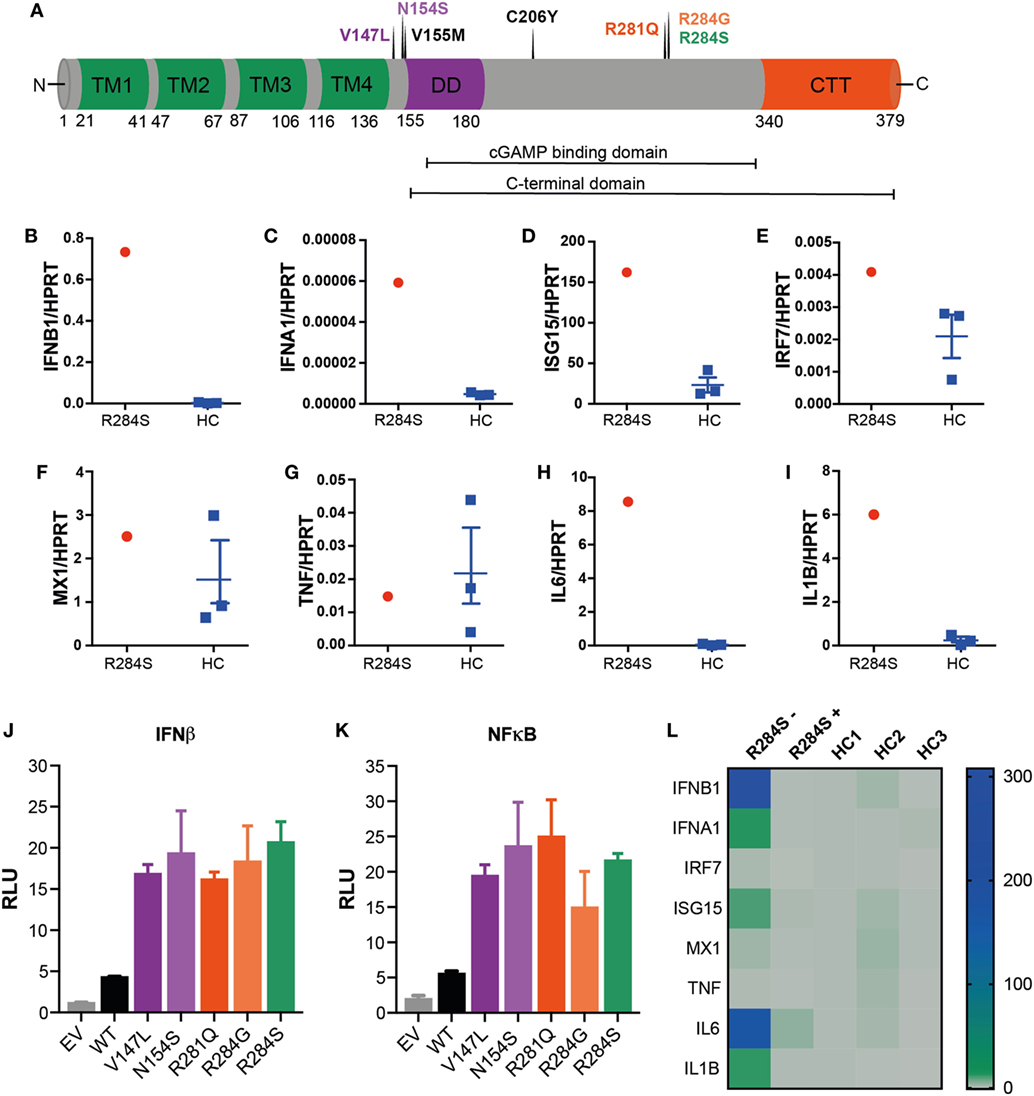
Figure 2. R284S is a pathogenic gain-of-function stimulator of interferon genes (STING) mutation. (A) Schematic structure of human STING: four transmembrane domains (TM, green), the dimerization domain (DD, purple), the C-terminal tail (CTT, red) region, and indicating the locations of known STING-associated vasculopathy with onset in infancy (SAVI) mutations. (B–I) Peripheral blood mononuclear cells (PBMCs) were isolated from the patient and three healthy controls (HC) and analyzed ex vivo for expression of inflammatory mediators by quantitative polymerase chain reaction (qPCR) analysis. Data for HC are shown as mean ± SEM of the three individuals. (J) IFNβ and (K) NF-κB luciferase reporter activity was monitored following transfection of HEK293T cells with an empty vector (EV) control, wild-type (WT) STING, or the indicated SAVI mutants of STING. Firefly luciferase reporter activity was normalized to the renilla control and presented as relative luciferase units (RLU). Data are representative of four independent experiments and shown as mean ± SD of three technical replicates. (L) Heatmap showing the mRNA expression of indicated inflammatory mediators as measured by qPCR analysis of PBMCs from the patient R284S before (−) or after (+) treatment with JAK inhibitor Ruxolitinib. This is compared to three HCs, with data shown relative to HC1.
We hypothesized the substitution of a serine for an arginine at position 284 in TMEM173 would result in constitutive activation of STING and consequently, autoinflammatory disease charactered by IFNαβ and NF-κB activation. To investigate this, peripheral blood mononuclear cells (PBMCs) were isolated from patient blood samples and analyzed for mRNA expression of ISGs and inflammatory cytokines by quantitative polymerase chain reaction (qPCR). Compared to healthy controls (HCs) our patient showed an IFN-gene signature with markedly raised levels of IFNB1 and the ISGs IFNA1, ISG15, IRF7, and MX1 supporting the diagnosis of an interferonopathy (Figures 2B–F). We also interrogated the mRNA expression of several NF-κB-dependent inflammatory cytokines: TNF, IL6, and IL1B. Interestingly, while TNF was not elevated, IL6 and IL1B were significantly upregulated (Figures 2G–I).
We next sought to directly determine if R284S triggers STING activation. STING expression plasmids encoding R284S and other known SAVI mutations (Figure 2A) were created by site-directed mutagenesis for in vitro studies. We then transfected HEK293T cells with wild-type (WT) and mutant versions of STING before assessing IFNβ and NF-κB activation using luciferase reporter systems. The R284S mutant STING induced high IFNβ activity compared to WT STING, similar to the previously published STING GoF mutants (Figure 2J). We further found that all SAVI mutants assayed, including R284S, induced markedly enhanced NF-κB activation compared to WT STING (Figure 2K). Therefore, in vitro analysis confirms R284S as a pathogenic GoF STING mutation.
After confirming the diagnosis of an interferonopathy, the patient was treated with a combination of oral prednisolone (2 mg/kg/day) and the JAK 1/2 inhibitor Ruxolitinib (5 mg daily) (12) which was well tolerated. His mobility and demeanor improved within days of steroid therapy including quality of sleep, respiratory effort, and cessation of his frequent vomiting episodes. His appetite improved, with weight gain of approximately 1.5 kg in 4 weeks. Serial weight velocity has subsequently increased from <3rd to 20th percentile in 5 months. His pulmonary pressures became sub-systemic within 8 weeks of sildenafil, steroids, and Ruxolitinib therapy. Initial steroid therapy coincided with a notable neurodevelopmental improvement. He made rapid gains in mobility (gross motor), self-feeding (fine motor), sleep/social interactions (personal social), and receptive speech (communication) compared to previous years while on intensive support. This correlated with a reduction in ISGs and inflammatory cytokines in the patient PBMCs following 6 months of treatment (Figure 2L). However, the lividinous rash persisted, and 6 months later the patient developed complete loss of his nasal septum while continuing on Ruxolitinib therapy. There was partial recrudescence of his gastrostomy feed related vomiting when his steroids were weaned. In summary, the treatment regime resulted in an excellent systemic response; however, it is possible that some tissue specific processes may remain uncontrolled.
Discussion
The clinical phenotype in almost all SAVI patients has included persistent systemic inflammation, cutaneous rash, failure to thrive, vasculitis of varying degrees, and interstitial lung disease (2–9). There are now two reports of pulmonary arterial hypertension in SAVI, presumed secondary to interstitial lung disease (4, 9). This may be the case in our patient, where a full work-up of the chest was not possible. However, our patient displays atypical features for SAVI, with no evidence of vasculitis or ongoing systemic inflammation, an atypical skin rash, and presentation with opportunistic infection, which suggested primary immunodeficiency. A different mutation at the same site as our patient, reported by Melki et al. (R284G) had interstitial lung disease but no significant inflammation, leading to suggestions of phenotypic differences between the two main sites of GoF mutations in STING (8).
Even more salient to the current case, while this manuscript was in preparation, Konno et al. described a 9-month-old infant with the same de novo heterozygous mutation in STING (p.R284S) (13). That patient presented without typical features of SAVI, but with a severe neck abscess, and later succumbed to pneumonia. In vitro studies conducted by Konno et al. agree with the data presented here that the p.R284S STING mutation is constitutively active. Significantly, we were able to confirm this finding through ex vivo analysis of patient cells. R284 is located within the cyclic dinucleotide binding domain of STING; however, its mutation does not seem to alter ligand affinity (8, 13). An alternate explanation could be that R284S changes/blocks the ubiquitination of STING at K289, which may increase its activation when stimulated (14), or that this region is required for appropriate shuttling of STING between the endoplasmic reticulum and Golgi (15). Overall, our study and that of Konno et al. provide more evidence that mutations outside the dimerization interface still cause GoF SAVI, but may present with atypical disease symptoms. A summary and comparison of the findings from all three patients with heterozygous GoF mutations in STING R284 is presented in Table 2.
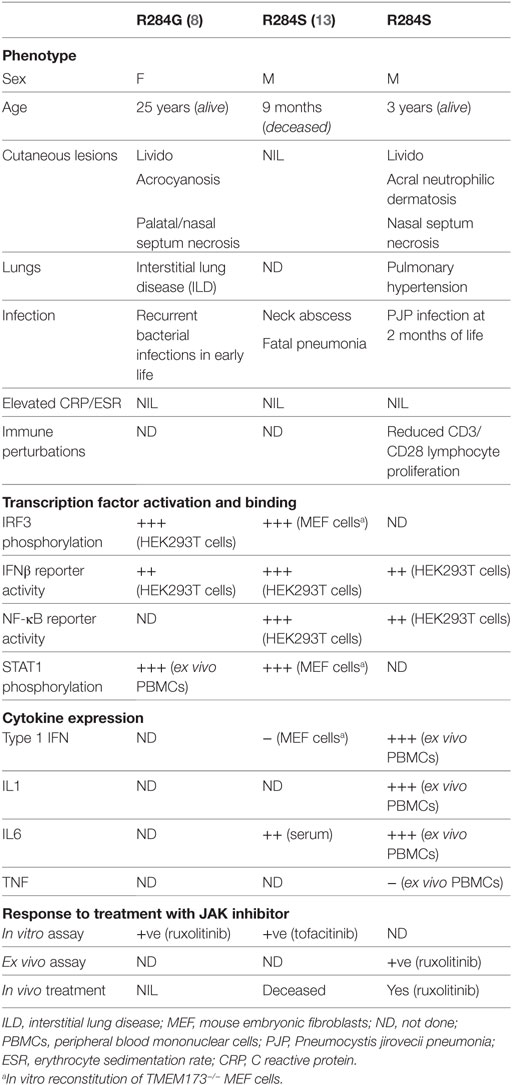
Table 2. Comparison of phenotypic changes associated with heterozygous mutation at p.R284 stimulator of interferon genes reported in the literature and this study.
Infections have not been a predominant part of SAVI, although patients may have recurrent bacterial upper respiratory infections (including pseudomonas infections), which is likely to be attributable to structural predisposition to infection from evolving interstitial lung disease rather than an immune defect (2–9). Patients have also demonstrated lymphopenia, a feature seen in the first STING GoF (N153S) mutant mouse (10). Recently, Bouis et al. characterized another heterozygous STING mutation in mice (V154M) which demonstrated a severe combined immunodeficiency phenotype affecting T/B/NK cell numbers (16). This was associated with an intrinsic defect in anti-CD3/CD28 T cell proliferation that was partially IFN dependent and correlates with our phenotypic observation of reduced anti-CD3/CD8 stimulated cell proliferation and immunodeficiency in our patient. From this perspective, it is noteworthy that our patient demonstrated a poor response to anti-CD3 but not to PHA stimulation in early life. An intriguing feature of our patient is that he never relapsed with Pneumocystis jirovecii pneumonia (PJP) after coming off co-trimoxazole, which would be unusual for a patient with a primary immunodeficiency predisposing to this organism, such as CD40L deficiency. Therefore, perhaps this predisposition reduced over time. In terms of a link between STING and PJP predisposition, type I IFNs are thought to play a role in the control of this fungus (17); however, linking constitutive STING activation to Pneumocystis infection remains speculative.
The STING N153S mutant mouse closely resembles some manifestations of SAVI such as splenomegaly, pleural effusions, respiratory distress, and ulcerative skin lesions (10). Interestingly, when crossed to mice deficient in IRF3 (preventing the production of IFNβ via STING) the mice still developed dysregulation of immune cells and lung disease. Therefore, NF-κB-dependent cytokines may play a dominant role in the phenotype observed for this SAVI mouse model. However, while the N153S mice developed chronic perivascular inflammation and organized thrombosis in pulmonary blood vessels, they did not develop the pulmonary fibrosis observed in SAVI and demonstrated a milder upregulation of the type I IFN signature compared to their human counterparts. This provides an interesting comparison to the atypical features observed in our patient with the R284S mutation. While the acute pulmonary hypertensive crisis was attributed to interstitial lung disease common to SAVI cases, the patient did not demonstrate clinical signs of lung parenchymal disease (tachypnea or hypoxemia). Moreover, his overnight pulse oximetry studies revealed a normal oxygen saturation range >92% in ambient room air. We surmise that the pulmonary hypertension in the absence of clinically evident lung disease might represent a primary vascular pathological process similar to that seen in the SAVI mouse model. Whether mutations in R284 can result in preferential, site-specific vasculitis given the absence of cutaneous vasculitis in our patient is unknown. It would be very interesting to know if a mouse model encoding a mutation analogous to R284S could reveal a different phenotype and help to explain why mutations outside the dimerization domain of human STING display features atypical of SAVI.
This case reinforces the atypical presentation of two previous patients with mutations at position R284 and raises the possibility that variants at this locus may present a different phenotype to patients with variants elsewhere in the STING protein. This is the first patient with a STING GoF mutation documented to suffer from PJP infection, which is an important consideration for SAVI patients who display lymphopenia and impaired T-cell proliferation. In vitro functional analysis of the R284S STING mutation found evidence of IFN and NF-κB activation, however, did not reveal a basis for potential genotype–phenotype correlation. Our study supports the benefit of JAK inhibition with Ruxolitinib for these patients, but raises the possibility that this intervention may provide incomplete control of some symptoms.
Methods
Patient and Study Approval
Written informed consent for publication of this case report was obtained from the patients guardians. The patient was consented for WGS under South East Sydney Local Health District ethics (HREC/11/POWH/152). The patient was recruited to the Australia Autoinflammatory Diseases Registry (AADRY, HREC/15/Mon/31) for ex vivo examination of PBMCs. HC PBMC were obtained from blood collected at the Volunteer Blood Donor Registry under Walter and Eliza Hall Institute of Medical Research HREC 10/02.
Whole Genome Sequencing
Whole genome sequencing was performed using the Illumina HiSeqX10 platform, and the Seave platform was used for bioinformatic analysis.
Lymphocyte Functional Assays
Anti-CD3, anti-CD28-, and PHA-stimulated lymphocyte proliferation assays were performed on whole blood, using uptake of tritiated thymidine, as a clinical test through SA Pathology, Adelaide, South Australia.
PBMC Isolation and mRNA Expression Analysis
Human PBMCs were purified from whole blood over a Ficoll density gradient (GE Healthcare) and lysed for RNA purification using RNeasy Plus Mini Kit (Qiagen) as directed by the manufacturer. RNA was reverse transcribed to cDNA using SuperScript III Reverse transcriptase (Thermo Fisher). qPCR was performed with Maxima SYBR Green master mix (Thermo Fisher) using primers designed for the specified genes. Expression of target genes was normalized to the expression of the housekeeping gene HPRT. Primer sequences used can be provided upon request.
Generation of STING Variants
To generate plasmids expressing STING carrying the SAVI mutations a human STING cDNA template (pEF-BOS-mCitrine-hSTING) was mutated using the QuikChange Lightning Mutagenesis Kit (Agilent Technologies). The following mutagenesis primers were used:
V147L, 5′-GCTGAGATCTCTGCATTGTGTGAAAAAGGG-3′;
N154S, 5′-GAAAAA GGGAATTTCAGCGTGGCCCATGGG-3′;
R281Q, 5′-GCTGGCTTTAGCCAGGAGGATAGGCTTGAGCAGGC-3′;
R284G; 5′-GGCTTTAGCCGGGAGGATGGGCTTGAGCAGGCC-3′;
R284S, 5′-GGCTTTAGCCGGGAGGAT AGC CTTGAGCAGGCC-3′.
Luciferase Assay
Approximately 1.5 × 105 HEK293T cells were seeded per well in a 24-well tissue culture plate. In each well 500 ng of expression plasmids were transiently transfected per well along with 100 ng of either an IFNβ or NF-κB firefly luciferase reporter plasmid and 10 ng renilla plasmid as an internal control. After 24 h, cells were lysed with Passive Lysis Buffer (Promega), and luminescence was measured on a luminometer using the Dual-Luciferase Reporter Assay System substrates (Promega). Conditions were performed in technical triplicates and firefly luciferase values were normalized to the renilla control.
Ethics Statement
The patient was consented for whole genome sequencing (WGS) under South East Sydney Local Health District ethics (HREC/11/POWH/152). The patient was recruited to the Australia Autoinflammatory Diseases Registry (AADRY, HREC/15/Mon/31) for ex vivo examination of PBMCs. Healthy control PBMC were obtained from blood collected at the Volunteer Blood Donor Registry under Walter and Eliza Hall Institute of Medical Research HREC 10/02.
Author Contributions
RS, BW, MW, RM, CL, MAW, VK, and PG were involved in collecting phenotypic data from the patient. KB, SD, FM, DD, and SM performed or analyzed ex vivo and in vitro experiments. CIRCA managed genomic sequencing. AADRY managed ex vivo analysis. RS, KB, SD, FM, DD, PG, and SM wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge: Jenni Harris for co-ordinating the Australian Autoinflammatory Diseases Registry and for the provision of healthy control samples through the Volunteer Blood Donor Registry; Prof. S. Kossard, Kossard Dermatopathologists, who kindly reviewed the skin biopsy; The Clinical Trials & Biorepository Team, St. Vincent’s Centre for Applied Medical Research for storage and handling of PBMC cells for ex vivo analysis.
Funding
SM acknowledges funding from NHMRC grants (1144282, 1142354, and 1099262), The Sylvia and Charles Viertel Foundation, HHMI-Wellcome International Research Scholarship, and Glaxosmithkline. SD acknowledges funding from NHMRC ECF: GNT1143412. Costco Wholesale Australia and the Fundraising department of The Children’s Hospital at Westmead provided generous support to enable genomic sequencing through a SCHN rare disease genomics initiative.
References
1. Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature (2008) 455:674–8. doi:10.1038/nature07317
2. Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Sanchez GAM, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med (2014) 371:507–18. doi:10.1056/NEJMoa1312625
3. Jeremiah N, Neven B, Gentili M, Callebaut I, Maschalidi S, Stolzenberg MC, et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest (2014) 124:5516–20. doi:10.1172/JCI79100
4. Omoyinmi E, Melo Gomes S, Nanthapisal S, Woo P, Standing A, Eleftheriou D, et al. Stimulator of interferon genes-associated vasculitis of infancy. Arthritis Rheumatol (2015) 67:808. doi:10.1002/art.38998
5. Chia J, Eroglu FK, Ozen S, Orhan D, Montealegre-Sanchez G, De Jesus AA, et al. Failure to thrive, interstitial lung disease, and progressive digital necrosis with onset in infancy. J Am Acad Dermatol (2016) 74:186–9. doi:10.1016/j.jaad.2015.10.007
6. Picard C, Thouvenin G, Kannengiesser C, Dubus JC, Jeremiah N, Rieux-Laucat F, et al. Severe pulmonary fibrosis as the first manifestation of interferonopathy (TMEM173 mutation). Chest (2016) 150:e65–71. doi:10.1016/j.chest.2016.02.682
7. Konig N, Fiehn C, Wolf C, Schuster M, Cura Costa E, Tungler V, et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann Rheum Dis (2017) 76:468–72. doi:10.1136/annrheumdis-2016-209841
8. Melki I, Rose Y, Uggenti C, Van Eyck L, Fremond ML, Kitabayashi N, et al. Disease-associated mutations identify a novel region in human STING necessary for the control of type I interferon signaling. J Allergy Clin Immunol (2017) 140:543–552e545. doi:10.1016/j.jaci.2016.10.031
9. Yu ZX, Zhong LQ, Song HM, Wang CY, Wang W, Li J, et al. [Stimulator of interferon genes-associated vasculopathy with onset in infancy: first case report in China]. Zhonghua Er Ke Za Zhi (2018) 56:179–85. doi:10.3760/cma.j.issn.0578-1310.2018.03.005
10. Warner JD, Irizarry-Caro RA, Bennion BG, Ai TL, Smith AM, Miner CA, et al. STING-associated vasculopathy develops independently of IRF3 in mice. J Exp Med (2017) 214:3279–92. doi:10.1084/jem.20171351
11. Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature (2016) 536:285–91. doi:10.1038/nature19057
12. Fremond ML, Rodero MP, Jeremiah N, Belot A, Jeziorski E, Duffy D, et al. Efficacy of the Janus kinase 1/2 inhibitor ruxolitinib in the treatment of vasculopathy associated with TMEM173-activating mutations in 3 children. J Allergy Clin Immunol (2016) 138:1752–5. doi:10.1016/j.jaci.2016.07.015
13. Konno H, Chinn IK, Hong D, Orange JS, Lupski JR, Mendoza A, et al. Pro-inflammation associated with a gain-of-function mutation (R284S) in the innate immune sensor STING. Cell Rep (2018) 23:1112–23. doi:10.1016/j.celrep.2018.03.115
14. Ni G, Konno H, Barber GN. Ubiquitination of STING at lysine 224 controls IRF3 activation. Sci Immunol (2017) 2(11):eaah7119. doi:10.1126/sciimmunol.aah7119
15. Dobbs N, Burnaevskiy N, Chen D, Gonugunta VK, Alto NM, Yan N. STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell Host Microbe (2015) 18:157–68. doi:10.1016/j.chom.2015.07.001
16. Bouis D, Kirstetter P, Arbogast F, Lamon D, Delgado V, Jung S, et al. Severe combined immunodeficiency in sting V154M/WT mice. J Allergy Clin Immunol (2018). doi:10.1016/j.jaci.2018.04.034
Keywords: stimulator of interferon genes, STING-associated vasculopathy with onset in infancy, interferon, NF-κB, JAK, immunodeficiency
Citation: Saldanha RG, Balka KR, Davidson S, Wainstein BK, Wong M, Macintosh R, Loo CKC, Weber MA, Kamath V, CIRCA, AADRY, Moghaddas F, De Nardo D, Gray PE and Masters SL (2018) A Mutation Outside the Dimerization Domain Causing Atypical STING-Associated Vasculopathy With Onset in Infancy. Front. Immunol. 9:1535. doi: 10.3389/fimmu.2018.01535
Received: 09 May 2018; Accepted: 21 June 2018;
Published: 06 July 2018
Edited by:
Seza Ozen, Hacettepe University, TurkeyReviewed by:
Helmut Wittkowski, Universitätsklinikum Münster, GermanyPui Y. Lee, Harvard University, United States
Copyright: © 2018 Saldanha, Balka, Davidson, Wainstein, Wong, Macintosh, Loo, Weber, Kamath, CIRCA, AADRY, Moghaddas, De Nardo, Gray and Masters. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul Edgar Gray, paul.gray1@health.nsw.gov.au;
Seth Lucian Masters, masters@wehi.edu.au
†These authors have contributed equally to this work.
 Rohit G. Saldanha1,2†
Rohit G. Saldanha1,2† Katherine R. Balka
Katherine R. Balka Sophia Davidson
Sophia Davidson Martin A. Weber
Martin A. Weber Fiona Moghaddas
Fiona Moghaddas Dominic De Nardo
Dominic De Nardo Paul Edgar Gray
Paul Edgar Gray Seth Lucian Masters
Seth Lucian Masters