Abstract
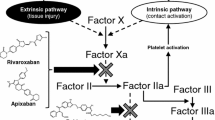
Background and Objective: Rivaroxaban is an oral, direct Factor Xa inhibitor, which is at an advanced stage of clinical development for prevention and treatment of thromboembolic disorders. Two phase II studies, ODIXa-DVT and EINSTEIN DVT, assessed the efficacy and safety of oral rivaroxaban (once daily or twice daily) for treatment of acute deep-vein thrombosis (DVT). Population pharmacokinetic and pharmacodynamic analyses of rivaroxaban in patients in these two phase II studies were conducted to characterize the pharmacokinetics/pharmacodynamics of rivaroxaban and the relationship between important patient covariates and model parameters. Exposure simulations in patients with atrial fibrillation (AF) were also performed in order to predict the exposure of rivaroxaban, using modified demographic data reflecting the characteristics of a typical AF population.
Methods: A population pharmacokinetic model was developed using plasma samples from these patients. Various simulations were conducted to explore the pharmacokinetics of rivaroxaban in patients with DVT and to predict exposure in those with AF. Correlations between plasma rivaroxaban concentrations and the prothrombin time, Factor Xa activity, HepTest® and activated partial thromboplastin time were also described.
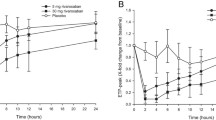
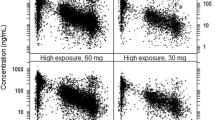
Results: The pharmacokinetics of rivaroxaban in patients with DVT were found to be consistent and predictable across all doses studied. The area under the plasma concentration-time curve (AUC) increased dose dependently. The same total daily doses given once daily achieved higher maximum plasma concentration (Cmax) values (∼20%) and lower trough (minimum) plasma concentration (Ctrough) values (∼60%) than when given twice daily; however, the 5th–95th percentile ranges for these parameters overlapped. Rivaroxaban clearance was moderately influenced by age and renal function, and the volume of distribution was influenced by age, body weight and sex; the effects were within the observed interindividual variability. Simulations in virtual patient populations with AF showed that a rivaroxaban dose of 15 mg once daily in patients with creatinine clearance of 30–49 mL/min would achieve AUC and Cmax values similar to those observed with 20 mg once daily in patients with normal renal function. The prothrombin time correlated almost linearly with plasma rivaroxaban concentrations (≤500 µg/L).
Conclusion: Population analyses of phase II clinical data indicated that the pharmacokinetics and pharmacodynamics of all rivaroxaban doses were predictable and were affected by expected demographic factors in patients with acute DVT.










Similar content being viewed by others
References
White RH. The epidemiology of venous thromboembolism. Circulation 2003 Jun; 107(23 Suppl. 1): I4–I8
Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost 2005 Aug; 3(8): 1611–7
Heit JA, Silverstein MD, Mohr DN, et al. The epidemiology of venous thromboembolism in the community. Thromb Haemost 2001 Jul; 86(1): 452–63
Snow V, Qaseem A, Barry P, et al. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2007 Feb; 146(3): 204–10
Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 2008 Jun; 133(6 Suppl.): 454S–545S
Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 2008 Jun; 133(6 Suppl.): 160S–198S
Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939 — an oral, direct Factor Xa inhibitor. J Thromb Haemost 2005 Mar; 3(3): 514–21
Lang D, Freudenberger C, Weinz C. In vitro metabolism of rivaroxaban — an oral, direct Factor Xa inhibitor — in liver microsomes and hepatocytes of rat, dog and man. Drug Metab Dispos 2009 May; 37(5): 1046–55
Weinz C, Schwarz T, Kubitza D, et al. Metabolism and excretion of rivaroxaban, an oral, direct Factor Xa inhibitor, in rats, dogs and humans. Drug Metab Dispos 2009 May; 37(5): 1056–64
Kubitza D, Becka M, Voith B, et al. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther 2005 Oct; 78(4): 412–21
Kubitza D, Becka M, Wensing G, et al. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939 — an oral, direct Factor Xa inhibitor — after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005 Dec; 61(12): 873–80
Mueck W, Eriksson BI, Bauer KA, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban — an oral, direct factor Xa inhibitor — in patients undergoing major orthopaedic surgery. Clin Pharmacokinet 2008 Mar; 47(3): 203–16
Mueck W, Borris LC, Dahl OE, et al. Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost 2008 Sep; 100(3): 453–61
Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008 Jun; 358(26): 2765–75
Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008 Jul; 372(9632): 31–9
Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008 Jun; 358(26): 2776–86
Turpie AGG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009 May; 373(9676): 1673–80
Agnelli G, Gallus A, Goldhaber SZ, et al. Treatment of proximal deep-vein thrombosis with the oral direct Factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-DVT (Oral Direct Factor Xa Inhibitor BAY 59-7939 in Patients with Acute Symptomatic Deep-Vein Thrombosis) study. Circulation 2007 Jul; 116(2): 180–7
Buller HR, Lensing AW, Prins MH, et al. A dose-ranging study evaluating once-daily oral administration of the Factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the EINSTEIN-DVT dose-ranging study. Blood 2008 Sep; 112(6): 2242–7
The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010 Dec; 363(26): 2499–510
Mueck W, Becka M, Kubitza D, et al. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban — an oral, direct Factor Xa inhibitor — in healthy subjects. Int J Clin Pharmacol Ther 2007 Jun; 45(6): 335–44
Beal SL, Sheiner LB. Estimating population kinetics. Crit Rev Biomed Eng 1982; 8(3): 195–222
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16(1): 31–41
Halperin JL; Executive Steering Committee, SPORTIF III and V Study Investigators. Ximelagatran compared with warfarin for prevention of thromboembolism in patients with nonvalvular atrial fibrillation: rationale, objectives, and design of a pair of clinical studies and baseline patient characteristics (SPORTIF III and V). Am Heart J 2003 Sep; 146(3): 431–8
Olsson SB; Executive Steering Committee of the SPORTIF III Investigators. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet 2003 Nov; 362(9397): 1691–8
Jones CA, McQuillan GM, Kusek JW, et al. Serum creatinine levels in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis 1998 Dec; 32(6): 992–9
Kubitza D, Becka M, Roth A, et al. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin 2008 Oct; 24(10): 2757–65
Clark B. Biology of renal aging in humans. Adv Ren Replace Ther 2000 Jan; 7(1): 11–21
Samama MM, Martinoli JL, Leflem L, et al. Assessment of laboratory assays to measure rivaroxaban — an oral, direct factor Xa inhibitor. Thromb Haemost 2010 Mar; 103(4): 815–25
Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA 2001 May; 285(18): 2370–5
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. Epub 2011 Aug 10
Bayer. Oral direct Factor Xa inhibitor rivaroxaban in patients with acute symptomatic pulmonary embolism with or without symptomatic deep-vein thrombosis: EINSTEIN-PE evaluation [ClinicalTrials.gov identifier NCT00439777]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov/show/NCT00439777 [Accessed 2011 Jul 1]
Acknowledgements
This analysis was supported by Bayer HealthCare, who held the data from the original studies and performed the analyses. Wolfgang Mueck, Anthonie Lensing and Frank Misselwitz are employees of Bayer HealthCare. Both Wolfgang Mueck and Frank Misselwitz own limited stock in Bayer AG. Hervé Decousus is a member of the study management and coordination committee for the EINSTEIN Study Programme and has received grants from Bayer HealthCare. Giancarlo Agnelli and Paolo Prandoni have no conflicts of interest to declare.
The authors would like to thank Dagmar Klein, Ulrike Krueger and Matthias Frede for their excellent technical assistance, and to acknowledge Shahid Salaria, who provided editorial assistance with funding from Bayer HealthCare and Johnson & Johnson Pharmaceutical Research & Development, LLC.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Mueck, W., Lensing, A.W.A., Agnelli, G. et al. Rivaroxaban. Clin Pharmacokinet 50, 675–686 (2011). https://doi.org/10.2165/11595320-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11595320-000000000-00000