Abstract
Objective
Respimat® Soft Mist™ Inhaler (SMI) is a novel, propellant-free device that significantly increases lung deposition compared with pressurised metered-dose inhalers (pMDIs). The aim of this study was to compare the efficacy and safety of ipratropium bromide/fenoterol hydrobromide (IB/FEN; Berodual®)delivered via Respimat® SMI and via a chlorofluorocarbon (CFC)-driven pMDI (CFC-MDI) in patients with asthma.
Design
Multicentre, randomised, double-blind, placebo-controlled, parallel-group study.
Patients
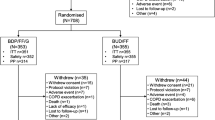
631 patients (18–65 years old) with stable asthma.
Interventions
After a 2-week run-in period (IB/FEN 20μg/50μg via CFC-MDI, two actuations four times a day), patients were randomised to 12 weeks’ treatment with one of five treatments: IB/FEN 10μg/25μg, 20μg/50μg or placebo via Respimat® SMI (one actuation four times a day), or IB/FEN 20μg/50μg or placebo via CFC-MDI (two actuations four times a day). The main efficacy measure was lung function (assessed on days 1, 29, 57 and 85); safety was assessed by monitoring adverse events.
Results
Bronchodilator responses to IB/FEN were much greater than those to placebo (mean peak increases in forced expiratory volume in 1 second [FEV1] on day 85: 0.498–0.521L, active treatment; 0.215 and 0.240L, placebo). According to the primary endpoint, i.e. the average change in FEV1 from test-day baseline over the 6 hours after dosing on day 85, neither IB/FEN dosage via Respimat® SMI was inferior to IB/FEN via pMDI (p < 0.001). Non-inferiority of the two Respimat® SMI dosages was supported by analyses of other lung function measures, e.g. average change in FEV1 from test-day baseline over the 6 hours after dosing on the other 3 test days, and peak FEV1 on all test days. Overall, the safety profile of IB/FEN via Respimat® SMI was comparable to that via CFC-MDI.
Conclusion
IB/FEN from Respimat® SMI is as effective and safe as from CFC-MDI and enables a 2-to 4-fold daily dose reduction of IB/FEN.







Similar content being viewed by others
Notes
The use of tradenames is for product identification purposes only and does not imply endorsement.
References
Pavia D. Efficacy and safety of inhalation therapy in chronic obstructive pulmonary disease and asthma. Respirology 1997; 2 Suppl. 1: S5–10
Duerden M, Price D. Training issues in the use of inhalers. Dis Manage Health Outcomes 2001; 9: 75–87
Lötvall J, O’Byrne P. Targeting drugs to the airways by different inhalation devices: role of deposition characteristics. Bio-drugs 1999; 12: 279–89
Zierenberg B, Eicher J, Dunne S, et al. Boehringer Ingelheim Nebulizer BINEB®: a new approach to inhalation therapy. Respir Drug Deliv 1996; 5: 187–93
Zierenberg B. Optimizing the in vitro performance of Respimat. J Aerosol Med 1999; 12 Suppl. 1: S19–24
Hochrainer D, Hölz H. Comparison of cloud velocities delivered from Respimat® soft mist inhaler or MDIs [abstract]. J Aerosol Med 2001; 14:386: P1–5
Newman SP, Steed KP, Reader SJ, et al. Efficient delivery to the lungs of flunisolide aerosol from a new portable hand-held multidose nebuliser. J Pharm Sci 1996; 85: 960–4
Steed KP, Towse LJ, Freund B, et al. Lung and oropharyngeal depositions of fenoterol hydrobromide delivered from the prototype III hand-held multidose Respimat® nebuliser. Eur J Pharm Sci 1997; 5: 55–61
Newman SP, Brown J, Steed KP, et al. Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of Respimat® with conventional metered-dose inhalers with and without spacer devices. Chest 1998; 113: 957–63
Littner M, Taylor JR, Ghafouri M, et al. A dose ranging study of ipratropium bromide solution delivered via a novel soft mist inhaler in patients with chronic obstructive pulmonary disease (COPD) [abstract]. Eur Respir J 2000; 16 Suppl. 31: 55S
Van Noord JA, Smeets JJ, Creemers JP, et al. Delivery of fenoterol via Respimat®, a novel ’soft mist’ inhaler. Respiration 2000; 67: 672-8
Goldberg J, Freund E, Beckers B, et al. Improved delivery of fenoterol plus ipratropium bromide using Respimat® compared with a conventional metered dose inhaler. Eur Respir J 2001; 17: 225–32
Frolund L, Madsen F, Svendsen UG, et al. Comparison of two aerosols containing both fenoterol and ipratropium in a high (Duovent) and low (Berodual) concentration, respectively. Respiration 1986; 50 Suppl. 2: 270–3
Philip-Joet F, Reynaud-Gaubert M, Jirou-Najou JL, et al. Comparison of Berodual and salbutamol in asthma: a multicenter evaluation. Respiration 1990; 57: 379–83
Kunkel G, Magnussen H, Bergmann K-C, et al. Respimat (a new Soft Mist Inhaler) delivering fenoterol plus ipratropium bromide provides equivalent bronchodilation at half the cumulative dose compared with a conventional metered dose inhaler in asthmatic patients. Respiration 2000; 67: 306–14
American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis 1987; 136: 225–44
Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report of working party standardisation of pulmonary lung function tests: European Community for Steel and Coal. Eur Respir J Suppl 1993; 16: 5–40
Iacono P, Velicitat P, Guemas E, et al. Improved delivery of ipratropium bromide using Respimat (a new soft mist inhaler) compared with a conventional metered dose inhaler: cumulative dose response study in patients with COPD. Respir Med 2000; 94: 490–5
Maesen FPV, van Noord JA, Smeets JJ, et al. Dose-range finding study comparing a new soft mist inhaler with a conventional metered dose inhaler (MDI) to deliver fenoterol in patients with asthma [abstract]. Eur Respir J 1997; 10 Suppl. 25: 128s
Maesen FPV, Greefhorst LPM, Smeets JJ, et al. Therapeutic equivalence of a novel HFA134a-containing metered-dose inhaler and the conventional CFC inhaler (Berodual®) for the delivery of a fixed combination of fenoterol/ ipratropium bromide. Respiration 1997; 64: 273–80
Hodder R, Dzyngel B, Fagan NM, et al. Comparison of the long-term efficacy and safety of ipratropium bromide (IB) delivered by a new soft mist inhaler (SMI) versus conventional metered dose inhaler (MDI) in COPD patients [abstract]. Eur Respir J 2001; 18 Suppl. 33: 69s
Kilfeather SA, Ponitz HH, Beck E, et al. Comparison of the efficacy and safety of ipratropium bromide (IB)/fenoterol hydrobromide (FEN) delivered by a new soft mist inhaler (SMI) or a conventional metered dose inhaler (MDI) in COPD patients [abstract]. Eur Respir J 2001; 18 Suppl. 33: 70s
Von Berg A, Jeena PM, Soemantri PA, et al. Efficacy and safety of ipratropium bromide plus fenoterol inhaled via Respimat® Soft Mist™ Inhaler versus a conventional metered dose inhaler plus spacer in children with asthma. Pediatr Pulmonol. In press
Miszkiel KA, Beasley R, Rafferty P, et al. The contribution of histamine release to bronchoconstriction provoked by inhaled benzalkonium chloride in asthma. Br J Clin Pharmacol 1988; 25: 157–63
Zhang YG, Wright WJ, Tam WK, et al. Effect of inhaled preservatives on asthmatic subjects: II. Benzalkonium chloride. Am Rev Respir Dis 1990; 141: 1405–8
Beasley R, Fishwick D, Miles JF, et al. Preservatives in nebulizer solutions: risks without benefit. Pharmacotherapy 1998; 18: 130–9
Acknowledgements
This study was sponsored by Boehringer Ingelheim. The sponsor and principal author declare that the study has not been biased as a result of the support so given.
The authors would like to thank their fellow investigators from Belgium, the Netherlands and South Africa for their help with this study:
Belgium: Dr JL Aumann, Dr P Driesen, Dr P Gris, Dr J Machiels, Dr H Slabbynck, Dr I Stappaerts, Dr J Verhaert and Prof. P Vermeire.
The Netherlands: Dr R Aalbers, Dr JPHM Creemers, Dr ME Eland, Dr WBM Evers, Dr SJM Gans, Dr APM Greefhorst, Dr AJ van Harreveld, Dr JLM van Helmond, Dr JHLM van Kasteren, Dr AF Kuipers, Dr J van Noord, Dr HR Pasma, Dr J Prins, Dr R Rammeloo, Dr AJM Schreurs, Dr H Sinnighe Damsté, Dr AP Sips, Dr PI van Spiegel and Dr J Westbroek.
South Africa: Prof. ED Bateman, Dr LG Herbst, Prof. J Joubert, Dr MC Kamdar, Dr U Lalloo, Dr M van der Linden, Dr J Terblanché, and Dr JJ Viljoen.
The authors would also like to acknowledge the help of the following co-investigators: Dr PM van der Berg, Dr EJFM ten Berge, Dr AMG Hendriks, Dr I Roussouw, Dr E Janssens, Dr PB Luursema, Dr H Nell, Dr NJJ Schlösser, Dr FMJ Simons, Dr HH Timmer, Dr MW Verheul, Dr NC van Walree and Dr HJ van der Woude.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vincken, W., Bantje, T., Middle, M.V. et al. Long-Term Efficacy and Safety of Ipratropium Bromide plus Fenoterol via Respimat® Soft Mist™ Inhaler (SMI) versus a Pressurised Metered-Dose Inhaler in Asthma. Clin. Drug Investig. 24, 17–28 (2004). https://doi.org/10.2165/00044011-200424010-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200424010-00003