Tracheal suspension by using 3-dimensional printed personalized scaffold in a patient with tracheomalacia
Introduction
Tracheomalacia (TM) is a condition of excessive collapse of the airway during respiration that can lead to life-threatening cardiopulmonary arrests (1,2). The congenital TM is often associated with tracheoesophageal fistula/esophageal atresia, bronchopulmonary dysplasia, and prematurity. Secondary TM occurs from external compression by vascular structures, tumors or cysts, or internal pressure caused by prolonged endotracheal intubation or tracheostomy (3). Mild and moderate TM tends to resolve over time with growth and development of a child patient (4,5). Severe cases need to be addressed because of significant morbidity and mortality (3).
Current therapies for severe TM include tracheostomy tube placement with prolonged mechanical ventilation, cardiovascular procedures to relieve compression from abnormal anatomy, and intraluminal airway stents. However, all these therapies have their instinct weakness, and the severe TM is lacking in an adequate intervention (6). Tracheostomy tube placement always accompanies tube occlusion with an incidence of 43%, which may cause respiratory arrest, especially for child patient (7). Aortopexy is the most common method for treatment of severe TM. However, it carries a high complication rate, including pericardial effusion, mediastinitis, and recurrence or regression of disease with cardiopulmonary arrest (8). Tracheal stenting can be performed without serious invasion, but migration of stenting and the recurrence of airway obstruction due to granulation tissue formation are another problem that can not be ignored (8).
Furthermore, the external tracheal stabilization techniques were attempted. Some experiments proved that the animals with external splinting were free from signs of respiratory distress post-operatively and no serious complication was found (9). However, the fixed-size external implants may restrict growth of trachea and inhibit natural improvement of the disease, and the premature splint degradation and loss of airway support are also the serious problems (9). Three-dimensional printing (3DP) arose from the automotive and aerospace industry in the 1980s and has subsequently been applied to customization of medical devices (10). 3DP has currently untapped potential to provide custom, protean devices for challenging and life-threatening disease processes. After meticulous design, we created a bioabsorbable external scaffold, which would support for at least 24 months, to maintain the native lumen size of collapsed airways. It allows transverse plane movement and normal cervical range of motion, but does not alter the mucociliary architecture of airway. After being placed extralumenally, the symptoms of the patient were improved remarkably, and there was no any complication.
Operative techniques
Characteristics of the patient
A 46-year-old female patient with tracheostenosis was referred to our department for treatment. The patient was diagnosed with endobronchial tuberculosis 2 years ago, and she received a regular antituberculosis therapy by taking isoniazid, rifampin, pyrazinamide and ethambutol. During the treatment, the patient suffered from dyspnea, and this symptom has developed gradually. The bronchoscope and chest computed tomography (CT) was subsequently performed, which showed a length of malacia in the trachea. Considering the development of the disease, a follow-up by using bronchoscope and chest CT was made every 3–6 months in the last 2 years, and the biopsy of trachea mucosa was also performed to detect the mycobacterium tuberculosis. Until no advancement of tracheomalacia had been found for 6 months, the further surgery was prepared.
Production of trachea scaffold
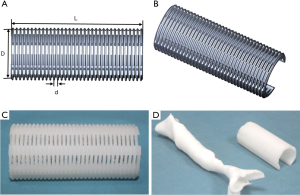
The scaffold was designed and made by Xi’an Jiaotong University. All of the parameters were set according to the CT value of tracheal malacic segment. As shown in the Figure 1A,B, the length (L) and external diameter (D) was 70 and 22 mm respectively. Moreover, the structure of the scaffold was projected as thread type with a pitch of 2 mm (d) and an open ring of 270°. For satisfying the biocompatibility and intensity, we selected the polycaprolactone [PCL, (C6H10O2)n] as the raw material of scaffold. The PCL was purchased from Daigang Biomaterial Co., Ltd (Jinan, China). The molecular weight was 80,000 units, and the intrinsic viscosity number was 0.5–1.0 dL/g. In theory, PCL with the molecular weight above 65,000 can stably exist 2 years in vivo, and then it will gradually degrade into H2O and CO2 (11). Since the acid environment after degradation can stimulate the hyperplasia and fibrosis of surrounding tissues, the malacic trachea can solidify gradually. The melting point was 58–64 °C. The 3DP technique of fused deposition modeling was applied with the melting temperature of 80 °C. Otherwise, we utilized 3DP polylactic acid tracheal model to evaluate the operative status of the malacic segment. Due to originating from the DICOM style file of thoracic CT, the 3DP model mimicked the malacic trachea. The products were showed in Figure 1C,D.
Surgical procedure
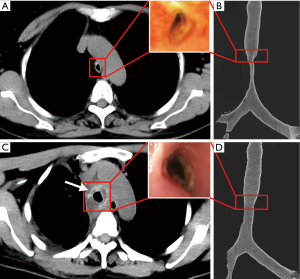
The tracheal malacic segment was assessed by chest CT and bronchoscope before (Figure 2A,B) and after (Figure 2C,D) the surgery. In the field of bronchoscope, the tracheal wall of malacic segment collapsed, especially in expiratory phase (Figure 2A). And the inner diameter of the narrowest cavity was only 0.3 cm. The three-dimensional reconstruction of CT images showed the tracheal malacic segment, which was about 1.5 cm above the carina of trachea (Figure 2B). The length of malacic segment was 6 cm, while the length of entire trachea was 11.5 cm. Thus, it is difficult for directly resection of the malacic segment and end to end tracheal anastomosis. After being evaluated by an experienced team completely, the suspension surgery was performed. The patient was intubated in the supine position, and then she was reversed in left lateral horizontal position and had a posterolateral thoracotomy in the fourth intercostal space.
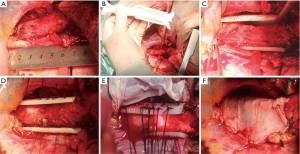
As shown in Figure 3A, the malacic trachea was split from other organs. The azygos vein was ligatured and cut because of the obstruction to malacic segment. Furthermore, the malacic segment was measured and compared with the 3DP tracheal model in surgery (Figure 3B). It can be clearly demonstrated that 3DP tracheal model is accurately matched with the actual malacic trachea. Then, the 3DP scaffold was placed around the malacic trachea (Figure 3C). Besides the complete separation of malacic trachea, the cooperation of anesthetist was the most important matter in the entire procedure. Because the intubation tube can not pass through the narrowest cavity, any operation in the malacic segment may result in the acute anoxia. The ventilatory pressure and capacity must be increased properly to maintain the oxygen saturation. Using 4-0 Polyglactin (Ethicon, Somerville, USA) sutures, we grasped and suspended the malacic trachea while bronchoscope was simultaneously performed to define and choose optimal points for suture placement precisely (Figure 3D). The sutures can be tied to the holes of scaffold, and at least four uniform positions were suspended in a cross section of malacic segment. The contiguous suspension positions in an axial direction were 0.5–1.0 cm apart (Figure 3E). At last, we put an artificial pleural patch around the 3DP scaffold to alleviate the abrasion to other organs, such as esophagus and precava (Figure 3F). After the accomplishment of whole surgery, the intubation tube with 6.5 mm passed through the malacic segment.
The patient was transferred into the intensive care unit after surgery, and she received assisted mechanical ventilator for 48 hours. In the first 24 hours, the intubation tube was put in the malacic segment to support the tracheal wall. And then we pulled out the tube above the malacic segment in the next 24 hours. After recovering autonomous respiration, the patient was extubated. The patient felt an obvious relief of dyspnea after surgery. She discharged from the hospital in 2 weeks after surgery. A detailed assessment about the efficacy and adverse reaction was performed. In Figure 2C,D, a remarkable improvement can be observed in the view of bronchoscope and chest CT. In the narrowest cavity of malacic trachea, the inner diameter increased from 0.3 to 1.0 cm, and the cross sectional area increased 4–5 times. In the chest CT, the scaffold can be clearly viewed (the white arrow in Figure 2C). In the follow-up of first 3 months, the patient had a remarkable improvement of breathing and physical strength. The chest CT images showed the scaffold was stable in the mediastinum, and the cavity of malacic trachea was the same as that in Figure 2C. No adverse reaction and toxicity were observed in the follow up.
Discussion
Many diseases, such as mediastinal lesion, tuberculosis, and other congenital factors, could compromise the blood and nutrient flow to the supporting cartilage, with the result of softening and collapse of the trachea wall (1-3). Anterior TM always occurs due to malformed tracheal cartilage, while posterior TM resulted from the membranous component. Currently there is no consensus regarding radiographic evaluation, standardized endoscopic evaluation, surgical approach, and medical treatments for TM (6). Multiple techniques have been created for the treatment of TM, however, there is currently no evidence supporting one therapy over another (6). The majority of methods are used to fix or stabilize the central airways and major bronchi, such as direct tracheobronchopexy, with either anterior suspension and/or posterior fixation for severe TM (12). The most common anterior tracheobronchopexy is aortopexy. Yet, a recent meta-analysis of aortopexy reported an overall complication rate of 15% and a mortality of 6%, even 4% of patients with worsening symptoms (13). This study suggested that there was room for improvement of tracheobronchopexy.
Many support biomaterials like mesh and sternal plate can be used to fix or stabilize the anterior cartilaginous or posterior membranous components (14). There are no specialized biomaterials for TM which must be casually fabricated by the doctors in operation. Thus, the support biomaterials with personalized design, good biocompatibility and sufficient mechanical strength should be developed for the tracheobronchopexy. In 2013, Zopf et al. (15) firstly created a 3DP scaffold to suspend the malformed bronchus. It must be worth noting that this PCL scaffold can not only satisfy the most requirements of tracheobronchopexy, but also biodegrade with the passage of time. This “4D materials” can make benefit to the child patient in growth period. In the following study of this group (16), another two patients with tracheobronchomalacia consecutively received bronchopexy by using the 3DP PCL scaffold. It has been proved the treatment efficacy, adverse reaction and biodegradation in the follow-up period. 3DP technique provided a novel tool to fabricate a personalized tracheal scaffold. We have used 3DP technique to make titanium sternum and ribs for reconstruction of chest wall previously (17). Furthermore, we continued to use the fused deposition modeling to make the PCL scaffold. The main lesion of TM was about 6 cm in the patient’s trachea which has reached the limitation of tracheal resection. And the primary endobronchial tuberculosis also prevented the traditional resection. After efficient treatment for endobronchial tuberculosis in 2 years, the suspension for malacic trachea was the optimal option. We designed the 3DP tracheal model and scaffold according to the tracheal CT images, which can make certain of the accuracy and matched-degree. As compared with the conventional materials, 3DP PCL scaffold can fix both the anterior and posterior tracheal wall. Meanwhile, the sufficient elasticity and intensity can support the compression from surrounding organs.
In a word, the 3DP PCL scaffold can supply a novel tool for suspending the malformed bronchus. But there are still some limitations that need to be addressed. Firstly, there is only one case in this research. The feasibility and safety of the scaffold must be proved in more samples. We made our best to select the uniform position to suspend the malacic trachea, but some triangles in a cross section were also observed. Maybe the structure of open ring is the main reason, and the surgical skills should be further improved. Moreover, more performance parameters, including materials, processing technic and structure design, must be discussed and confirmed for patients at different ages. Otherwise, it must be worth noting that the 3DP technique is difficult to be approved by FDA and CFDA. Because the 3DP product is individual and personalized for the specific patient, it is difficult to quantify and batch. This is also the common characteristic that all 3DP products must face.
Acknowledgements
Funding: This work was supported by key program of Clinical Research Innovation Fund and Young Talent Fund of Tangdu Hospital, the Fourth Military Medical University, China, and the National Natural Science Foundation of China (81370115, 51422508, 51675412).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology 2006;11:388-406. [Crossref] [PubMed]
- Boogaard R, Huijsmans SH, Pijnenburg MW, et al. Tracheomalacia and bronchomalacia in children: incidence and patient characteristics. Chest 2005;128:3391-7. [Crossref] [PubMed]
- Carden KA, Boiselle PM, Waltz DA, et al. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest 2005;127:984-1005. [Crossref] [PubMed]
- Cogbill TH, Moore FA, Accurso FJ, et al. Primary tracheomalacia. Ann Thorac Surg 1983;35:538-41. [Crossref] [PubMed]
- Jacobs IN, Wetmore RF, Tom LW, et al. Tracheobronchomalacia in children. Arch Otolaryngol Head Neck Surg 1994;120:154-8. [Crossref] [PubMed]
- Goyal V, Masters IB, Chang AB. Interventions for primary (intrinsic) tracheomalacia in children. Cochrane Database Syst Rev 2012;10:CD005304. [PubMed]
- Berry JG, Graham RJ, Roberson DW, et al. Patient characteristics associated with in-hospital mortality in children following tracheotomy. Arch Dis Child 2010;95:703-10. [Crossref] [PubMed]
- Valerie EP, Durrant AC, Forte V, et al. A decade of using intraluminal tracheal/bronchial stents in the management of tracheomalacia and/or bronchomalacia: is it better than aortopexy? J Pediatr Surg 2005;40:904-7; discussion 907. [Crossref] [PubMed]
- Zopf DA, Flanagan CL, Wheeler M, et al. Treatment of severe porcine tracheomalacia with a 3-dimensionally printed, bioresorbable, external airway splint. JAMA Otolaryngol Head Neck Surg 2014;140:66-71. [Crossref] [PubMed]
- Gross BC, Erkal JL, Lockwood SY, et al. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem 2014;86:3240-53. [Crossref] [PubMed]
- Sun H, Mei L, Song C, et al. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006;27:1735-40. [Crossref] [PubMed]
- Yokoi A, Arai H, Bitoh Y, et al. Aortopexy with tracheal reconstruction for postoperative tracheomalacia in congenital tracheal stenosis. J Pediatr Surg 2012;47:1080-3. [Crossref] [PubMed]
- Torre M, Carlucci M, Speggiorin S, et al. Aortopexy for the treatment of tracheomalacia in children: review of the literature. Ital J Pediatr 2012;38:62. [Crossref] [PubMed]
- Liu Z, Yang R, Shao F, et al. Controlled Trachea Suspension for Tracheomalacia After Resection of Large Anterior Mediastinal Mass. Ann Thorac Surg 2015;99:2225-7. [Crossref] [PubMed]
- Zopf DA, Hollister SJ, Nelson ME, et al. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med 2013;368:2043-5. [Crossref] [PubMed]
- Morrison RJ, Hollister SJ, Niedner MF, et al. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci Transl Med 2015;7:285ra64. [Crossref] [PubMed]
- Wang L, Cao T, Li X, et al. Three-dimensional printing titanium ribs for complex reconstruction after extensive posterolateral chest wall resection in lung cancer. J Thorac Cardiovasc Surg 2016;152:e5-7. [Crossref] [PubMed]


