-
PDF
- Split View
-
Views
-
Cite
Cite
Timothy F. Brewer, Preventing Tuberculosis with Bacillus Calmette-Guérin Vaccine: A Meta-Analysis of the Literature, Clinical Infectious Diseases, Volume 31, Issue Supplement_3, September 2000, Pages S64–S67, https://doi.org/10.1086/314072
Close - Share Icon Share
Abstract
This article reviews a previously published meta-analysis of 1264 titles or abstracts and 70 selected studies for evaluation of the efficacy of bacillus Calmette-Guérin (BCG) vaccine in preventing tuberculosis. Following review, data from 26 studies were included in the analysis. These 26 studies reveal that vaccination with BCG significantly reduces the risk of tuberculosis by an average of 50%. This level of protection persists across a number of subgroups defined by age at vaccination and study design. Vaccination with BCG was significantly associated with a reduction in the incidence of pulmonary tuberculosis and extrapulmonary disease. In general, the results of this meta-analysis lend weight and confidence to arguments favoring the use of BCG vaccine.
BCG is a well-studied vaccine. A recent review of the medical literature revealed hundreds of prospective clinical trials, case-control studies, and review articles spanning 46 years [1]. The trials examined the efficacy of BCG vaccine in different population groups, including infants, school-age children, and adults, as well as in various ethnic groups. Unfortunately, some trials have yielded different or contradictory findings, the most contradictory of which is a BCG efficacy rate that ranges from 0% to 80% for protective benefit. These discrepancies have fueled the ongoing controversy about the efficacy of BCG vaccine for preventing tuberculosis.
Questions about BCG vaccination that remain unresolved involve its overall efficacy, the duration of protective immunity, and how age at vaccination affects protection. Clinicians also need more consistent information on the efficacy of different strains of BCG vaccine.
The following meta-analysis by Colditz et al. [1] was undertaken to test the hypothesis that rates of tuberculosis are not different in BCG-vaccinated and BCG-nonvaccinated control populations and to explore the reasons for reported variation in protection rate among the published studies.
Methods
Specifically, the meta-analysis was designed to answer the following questions. (1) Is BCG vaccine efficacious? (2) How efficacious is BCG vaccine? (3) How confident can clinicians be when reviewing the results of these trials? (4) What factors are associated with better or worse performance of BCG vaccine? (5) What is the effect of age on vaccine efficacy?
The researchers reviewed 1264 articles or abstracts in 7 different languages and selected 70 trials for possible inclusion in a meta-analysis. Of the 70 reports selected, 14 prospective trials and 12 case-control studies met the inclusion and/or exclusion criteria.
Only studies measuring the efficacy of BCG vaccination in preventing tuberculosis cases and/or deaths were included in the meta-analysis. Included trials had to have a defined method of allocation between vaccine and control groups. This allocation method had to create study groups and control groups that were comparable in their risk of tuberculosis. Equivalent surveillance and follow-up of both groups were needed in order for the trial to be included in the meta-analysis.
Because these trials were conducted over a period of 46 years, clinical investigators used different methods to allocate study subjects to vaccine and control groups. The meta-analysis team divided selected trials into groups that used a randomized, alternate, or systematic process to allocate study subjects. In the alternate allocation process, every other individual was assigned to a specific group. In the systematic allocation system, individuals were stratified by age or school grade into vaccine or control groups.
To be included in the meta-analysis, case-control studies had to define the case patients and control subjects and to describe how BCG recipients in these 2 groups were identified. If control subjects were identified from a tuberculin-screened population and case patients were not, the study was excluded.
Data extracted from the trials included study design, age range, number of patients, outcomes, and cases or deaths, as well as variables that might have an impact on vaccine efficacy. The trials and studies were scored for their potential biases by means of a predetermined measurement of the validity of the study's design. Factors used in the assessment of the trials for potential bias included the number of participants lost to follow-up, the methods used to diagnosis tuberculosis, and whether tuberculosis diagnosis was made blind to treatment status. Case-control studies were assessed with regard to whether the determination of the exposure, or BCG status, was made blind to case or control status and the methods used to determine the diagnosis of tuberculosis, as well as other factors.
Information on vaccine efficacy from the clinical trials and case-control studies was combined separately by means of the DerSimonian and Laird random-effects method [2]. This method takes into account study size, within-study variation, and between-study variation. Summary estimates of the RR and OR were obtained for both the clinical trials and the case-control studies. With this method, a value <1 means that BCG is protective against tuberculosis. Protective effect was defined as 1 - RR. Because tuberculosis is a relatively rare outcome, the OR provides a close approximation of the RR.
A second random-effects regression method was developed to explore reasons for the heterogeneity in reported trial results. Previous literature was used to develop a set of variables that were examined in this model. Among the variables assessed were methodological validity of the included studies, latitude, vaccine strain, and age range of the population.
Results
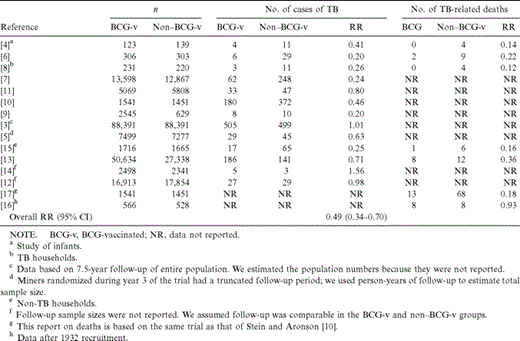
Data from the 7 trials [3–9] that used random allocation yielded an RR for tuberculosis of 0.37 (95% CI, 0.18–0.74) among those vaccinated with BCG, equivalent to a protective effect of 63%. When the 2 trials that used alternate allocation [10, 11] and the 4 trials that used systematic allocation [12–15] were added to this group, the RR for tuberculosis was 0.49 (95% CI, 0.34–0.70), equivalent to a protective effect of 51%.
In reports on the 7 trials in which deaths from tuberculosis were studied [4, 6, 8, 13, 15–17], the combined RR for death due to tuberculosis among those vaccinated with BCG was 0.29 (95% CI, 0.16–0.53), which shows that BCG vaccine has a 71% protective effect against death due to tuberculosis (table 1).
Reported data from clinical trials included in the meta-analysis providing estimated efficacy of bacillus Calmette-Guérin (BCG) vaccine against tuberculosis (TB) and TB-related death.
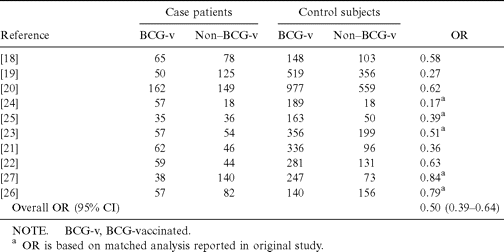
Analysis of the 8 case-control studies involving populations vaccinated as infants [18–25] showed a combined OR of 0.45 (95% Cl, 0.34–0.59), for a protective effect of 55% against tuberculosis. When the 10 case-control studies [18–27] with 1414 cases of predominantly pulmonary tuberculosis were combined (table 2), BCG vaccination had a 50% protective effect compared with no vaccination (95% CI for the OR, 0.39–0.64). The protective effect of BCG was stronger (83%) when the analysis was limited to patients in case-control studies [19, 24, 25] in which the diagnosis of tuberculosis was established by a positive culture or histology (95% CI for OR, 0.07–0.42).
Total nos. of tuberculosis cases in 10 case-control studies of bacillus Calmette-Guérin (BCG) vaccine efficacy.
Reports of 5 case-control studies conducted in populations vaccinated as infants [19, 20, 23, 28, 29] described results for cases of tuberculous meningitis, including 2 that reported only on meningitis. Among 181 cases of meningitis, BCG vaccination had a protective effect of 64% (95% CI for OR, 0.18–0.70).
A 2-covariate, random-effects regression model was used to explore variation in BCG efficacy among 13 prospective trials from which tuberculosis cases were reported. Geographic latitude and study validity score explained 66% of the between-study variation in the trials [3, 4, 5–15]. The efficacy of BCG vaccination increased with increasing distance from the equator and with higher study validity. In exploring the variation in vaccine efficacy reported from the case-control studies, only the methodological validity score explained a substantial amount (36%) of the between-study variation.
Different strains of BCG were not consistently associated with more favorable or less favorable results in the trials. Different BCG preparations and strains used in the same population gave similar levels of protection. Genetically identical BCG preparations yielded different results in different populations.
Conclusions
The meta-analysis of data from 14 prospective trials and 12 case-control studies of BCG efficacy indicate that vaccination with BCG significantly reduces the risk of tuberculosis, by an average of 50%. This level of protection persists across a number of subgroups of the studies defined by age at vaccination and study design. Age at vaccination was not a significant predictor of BCG efficacy. More than 85% of the tuberculosis cases in the prospective trials were classified by location or type, with 85% of the cases among BCG recipients and controls described as pulmonary. Therefore, vaccination with BCG was significantly associated with a reduction in pulmonary tuberculosis and extrapulmonary disease.
Speculation about the reasons for differences in BCG efficacy include the use of different strains of BCG, prevalence of other mycobacterial infections in the study population, genetic or age differences in the study population, reduced virulence of some of the Mycobacterium tuberculosis strains, and variation in BCG protection against different forms of tuberculosis [3, 12, 30]. Methodological flaws producing biases in the different trials have also been suggested to account for some of the variation in observed efficacy [31]. With use of random-effects regression methods to explore reasons for the variation in BCG efficacy reported from the trials, latitude and the methodological or study validity score explained a considerable amount of the heterogeneity noted. The study validity score also explained a substantial amount of the variation in vaccine efficacy noted in the case-control studies. Age at vaccination, in contrast, did not explain much of the variation in vaccine efficacy reported from the trials.
In general, the results of this meta-analysis lend weight and confidence to arguments favoring the use of BCG vaccine.
References
Author notes
Data previously published in The Journal of the American Medical Association (1994; 271:698–702).





Comments