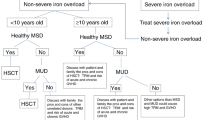
Summary:
For the past two decades, hematopoietic cell transplantation (HCT) has been used as effective therapy for selected inherited metabolic diseases (IMD) including Hurler (MPS IH) and Maroteaux–Lamy (MPS VI) syndromes, childhood-onset cerebral X-linked adrenoleukodystrophy (X-ALD), globoid-cell leukodystrophy (GLD), metachromatic leukodystrophy (MLD), α-mannosidosis, osteopetrosis, and others. Careful pre-HCT evaluation is critical and coordinated, multidisciplinary follow-up is essential in this field of transplantation. The primary goals of HCT for these disorders have been to promote long-term survival with donor-derived engraftment and to optimize the quality of life. Guidelines for HCT and monitoring are provided; a brief overview of long-term results is also presented.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fratantoni JC, Hall CW, Neufeld EF . The defect in Hurler and Hunter syndromes. II. Deficiency of specific factors involved in mucopolysaccharide degradation. Proc Natl Acad Sci USA 1969; 64: 360–366.
Di Ferrante N, Nichols BL, Donnelly PV et al. Induced degradation of glycosaminoglycans in Hurler's and Hunter's syndromes by plasma infusion. Proc Natl Acad Sci USA 1971; 68: 303–307.
Knudson Jr AG, Di Ferrante N, Curtis JE . Effect of leukocyte transfusion in a child with type II mucopolysaccharidosis. Proc Natl Acad Sci USA 1971; 68: 1738–1741.
Hobbs JR, Hugh-Jones K, Barrett AJ et al. Reversal of clinical features of Hurler's disease and biochemical improvement after treatment by bone-marrow transplantation. Lancet 1981; 2: 709–712.
Krivit W, Shapiro EG, Peters C et al. Hematopoietic stem-cell transplantation in globoid-cell leukodystrophy. N Engl J Med 1998; 338: 1119–1126.
Shapiro E, Krivit W, Lockman L et al. Long-term effect of bone-marrow transplantation for childhood-onset cerebral X-linked adrenoleukodystrophy. Lancet 2000; 356: 713–718.
Stillman AE, Krivit W, Shapiro E et al. Serial MR after bone marrow transplantation in two patients with metachromatic leukodystrophy. Am J Neuroradiol 1994; 15: 1929–1932.
Peters C, Balthazor M, Shapiro EG et al. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood 1996; 87: 4894–4902.
Peters C, Shapiro EG, Anderson J et al. Hurler syndrome: II. Outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. The Storage Disease Collaborative Study Group. Blood 1998; 91: 2601–2608.
Krivit W, Pierpont ME, Ayaz K et al. Bone-marrow transplantation in the Maroteaux-Lamy syndrome (mucopolysaccharidosis type VI). Biochemical and clinical status 24 months after transplantation. N Engl J Med 1984; 311: 1606–1611.
Krivit W . Maroteaux–Lamy syndrome (Mucopolysaccharidosis type VI): treatment by allogeneic bone marrow transplantation in 6 patients and potential for autotransplantation bone marrow gene insertion. Int Pediatr 1992; 7: 47.
Yamada Y, Kato K, Sukegawa K et al. Treatment of MPS VII (Sly disease) by allogeneic BMT in a female with homozygous A619V mutation. Bone Marrow Transplant 1998; 21: 629–634.
Krivit W, Sung JH, Shapiro EG et al. Microglia: the effector cell for reconstitution of the central nervous system following bone marrow transplantation for lysosomal and peroxisomal storage diseases. Cell Transplant 1995; 4: 385–392.
Field RE, Buchanan JA, Copplemans MG et al. Bone-marrow transplantation in Hurler's syndrome. Effect on skeletal development. J Bone Jt Surg Br 1994; 76: 975–981.
Neufeld E, Muenzer J . The Mucopolysaccharidoses. In: Schriver CR, Beaudet AL, Sly WS, Walle W (eds). The Metabolic and Molecular Bases of Inherited Disease, 8th edn. McGraw-Hill: New York, 2001, pp 3421–3452.
Summers CG, Purple RL, Krivit W et al. Ocular changes in the mucopolysaccharidoses after bone marrow transplantation A preliminary report. Ophthalmology 1989; 96: 977–984. Discussion 984–985.
Resnick JM, Krivit W, Snover DC et al. Pathology of the liver in mucopolysaccharidosis: light and electron microscopic assessment before and after bone marrow transplantation. Bone Marrow Transplant 1992; 10: 273–280.
Resnick JM, Whitley CB, Leonard AS et al. Light and electron microscopic features of the liver in mucopolysaccharidosis. Hum Pathol 1994; 25: 276–286.
Whitley CB, Ramsay NK, Kersey JH et al. Bone marrow transplantation for Hurler syndrome: assessment of metabolic correction. Birth Defects Orig Artic Ser 1986; 22: 7–24.
Malone BN, Whitley CB, Duvall AJ et al. Resolution of obstructive sleep apnea in Hurler syndrome after bone marrow transplantation. Int J Pediatr Otorh 1988; 15: 23–31.
Hugh-Jones K, Hobbs JR, Vellodi A et al. Long-term follow-up of children with Hurler's disease treated with bone marrow transplantation. In: Hobbs JR (ed.). Correction of Certain Genetic Diseases by Transplantation 1989. Headstart Printing: London, 1989, pp. 103–111.
Whitley CB, Belani KG, Chang PN et al. Long-term outcome of Hurler syndrome following bone marrow transplantation. Am J Med Genet 1993; 46: 209–218.
Krivit W, Sung JH, Lockman L et al. Bone marrow transplantation for the treatment of lysosomal and peroxisomal diseases: focus on central nervous system reconstitution. In: Rich RR, Fleisher TA, Schwartz BD (eds). Principles of Clinical Immunology. Mosby: St. Louis, 1995, pp. 1852.
Krivit W, Lockman LA, Watkins PA et al. The future for treatment by bone marrow transplantation for adrenoleukodystrophy, metachromatic leukodystrophy, globoid cell leukodystrophy and Hurler syndrome. J Inherit Metab Dis 1995; 18: 398–412.
Braunlin EA, Hunter DW, Krivit W et al. Evaluation of coronary artery disease in the Hurler syndrome by angiography. Am J Cardiol 1992; 69: 1487–1489.
du Cret RP, Weinberg EJ, Jackson CA et al. Resting Tl-201 scintigraphy in the evaluation of coronary artery disease in children with Hurler syndrome. Clin Nucl Med 1994; 19: 975–978.
Braunlin EA, Rose AG, Hopwood JJ et al. Coronary artery patency following long-term successful engraftment 14 years after bone marrow transplantation in the Hurler syndrome. Am J Cardiol 2001; 88: 1075–1077.
Masterson EL, Murphy PG, O'Meara A et al. Hip dysplasia in Hurler's syndrome: orthopaedic management after bone marrow transplantation (see comments). J Pediatr Orthop 1996; 16: 731–733.
Odunusi E, Peters C, Krivit W et al. Genu valgum deformity in Hurler syndrome after hematopoietic stem cell transplantation: correction by surgical intervention. J Pediatr Orthop 1999; 19: 270–274.
Van Heest AE, House J, Krivit W et al. Surgical treatment of carpal tunnel syndrome and trigger digits in children with mucopolysaccharide storage disorders. J Hand Surg (Am) 1998; 23: 236–243.
Krivit W, Shapiro EG, Balthazor M et al. Hurler syndrome: outcomes and planning following bone marrow transplantation. In: Stewards C, Hobbs JR (eds). Correction of Genetic Diseases by Transplantation III. Oxbridge Press Ltd: London, 1995, pp. 25–40.
Stauffer NR, Braunlin E, Whitley CB et al. Echocardiographic follow-up of Hurler syndrome after bone marrow transplantation. Circulation 1991; vol 84 (suppl II):462a.
Guffon N, Souillet G, Maire I et al. Follow-up of nine patients with Hurler syndrome after bone marrow transplantation (see comments). J Pediatr 1998; 133: 119–125.
Hoogerbrugge PM, Brouwer OF, Bordigoni P et al. Allogeneic bone marrow transplantation for lysosomal storage diseases. The European Group for Bone Marrow Transplantation. Lancet 1995; 345: 1398–1402.
Vellodi A, Young EP, Cooper A et al. Bone marrow transplantation for mucopolysaccharidosis type I: experience of two British centres. Arch Dis Child 1997; 76: 92–99.
Grewal SS, Krivit W, Defor TE et al. Outcome of second hematopoietic cell transplantation in Hurler syndrome. Bone Marrow Transplant 2002; 29: 491–496.
Jacobson P, Park JJ, DeFor TE et al. Oral busulfan pharmacokinetics and engraftment in children with Hurler syndrome and other inherited metabolic storage diseases undergoing hematopoietic cell transplantation. Bone Marrow Transplant 2001; 27: 855–861.
Kakkis ED, Muenzer J, Tiller GE et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med 2001; 344: 182–188.
Vellodi A, Young E, Cooper A et al. Long-term follow-up following bone marrow transplantation for Hunter disease. J Inherit Metab Dis 1999; 22: 638–648.
Imaizumi M, Gushi K, Kurobane I et al. Long-term effects of bone marrow transplantation for inborn errors of metabolism: a study of four patients with lysosomal storage diseases. Acta Paediatr Jpn 1994; 36: 30–36.
Bergstrom SK, Quinn JJ, Greenstein R et al. Long-term follow-up of a patient transplanted for Hunter's disease type IIB: a case report and literature review. Bone Marrow Transplant 1994; 14: 653–658.
McKinnis EJ, Sulzbacher S, Rutledge JC et al. Bone marrow transplantation in Hunter syndrome. J Pediatr 1996; 129: 145–148.
Shapiro EG, Lockman LA, Balthazor M et al. Neuropsychological outcomes of several storage diseases with and without bone marrow transplantation. J Inherit Metab Dis 1995; 18: 413–429.
Klein KA, Krivit W, Whitley CB . Poor cognitive outcome of eleven children with Sanfillipo syndrome after bone marrow transplantation and successful engraftment. Bone Marrow Transplant 1995; 15: S176.
Vellodi A, Young E, New M et al. Bone marrow transplantation for Sanfilippo disease type B. J Inherit Metab Dis 1992; 15: 911–918.
Bordigoni P, Vidailhet M, Lena M et al. Bone marrow transplantation for Sanfilippo syndrome. In: Hobbs JR (ed.), Correction of Certain Genetic Diseases by Transplantation 1989, Headstart Printing: London, 1989, pp. 114–119.
Moser HW, Smith KD, Watkins PA et al. X-Linked adrenoleukodystrophy. In: Schriver CR, Beaudet AL, Sly WS, Valle D (eds). The Metabolic and Molecular Bases of Inherited Disease, 8th edn. McGraw-Hill, New York, 2001, pp. 3257–3302.
Moser HW . Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain 1997; 120(8): 1485–1508.
Ronghe MD, Barton J, Jardine PE et al. The importance of testing for adrenoleucodystrophy in males with idiopathic Addison's disease. Arch Dis Child 2002; 86: 185–189.
Loes DJ, Hite S, Moser H et al. Adrenoleukodystrophy: a scoring method for brain MR observations. Am J Neuroradiol 1994; 15: 1761–1766.
Eichler FS, Barker PB, Cox C et al. Proton MR spectroscopic imaging predicts lesion progression on MRI in X-linked adrenoleukodystrophy. Neurology 2002; 58: 901–907.
Izquierdo M, Adamsbaum C, Benosman A et al. MR spectroscopic imaging of normal-appearing white matter in adrenoleukodystrophy. Pediatr Radiol 2000; 30: 621–629.
Pouwels PJ, Kruse B, Korenke GC et al. Quantitative proton magnetic resonance spectroscopy of childhood adrenoleukodystrophy. Neuropediatrics 1998; 29: 254–264.
Rajanayagam V, Balthazor M, Shapiro EG et al. Proton MR spectroscopy and neuropsychological testing in adrenoleukodystrophy. Am J Neuroradiol 1997; 18: 1909–1914.
Shapiro E, Lockman L, Balthazor M . Neurospychological and neurological function and quality-of-life before and after bone marrow transplantation for adrenoleukodystrophy. In: Ringden O, Hobbs JR, Stewards C (eds). Correction of Genetic Diseases by Transplantation IV. The COGENT Press: Middlesex, 1997, pp. 52–62.
Loes DJ, Stillman AE, Hite S et al. Childhood cerebral form of adrenoleukodystrophy: short-term effect of bone marrow transplantation on brain MR observations. Am J Neuroradiol 1994; 15: 1767–1771.
Aubourg P, Blanche S, Jambaque I et al. Reversal of early neurologic and neuroradiologic manifestations of X-linked adrenoleukodystrophy by bone marrow transplantation. N Engl J Med 1990; 322: 1860–1866.
Peters C, Abel S, Defor TE et al. The worldwide hematopoietic cell transplant experience for childhood onset cerebral X-linked adrenoleukodystrophy (COCALD). Blood 2000; 96: 842 (Abstr. 3638).
Wenger DA, Suzuki K, Suzuki Y et al. Galactosylceramide lipidosis: globoid cell leukodystrophy (Krabbe disease). In: Schriver CR, Beaudet AL, Sly WS, Valle D (eds). The Metabolic and Molecular Bases of Inherited Disease, 8th edn. McGraw-Hill: New York; 2001, pp. 3669–3694.
Kurtzberg J, Richards K, Wenger D et al. Correction of Krabbe disease with neonatal hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2002; 8: 97 (Abstr. 130).
von Figura K, Gieselman V, Jaeken J . Metachromatic leukodystrophy. In: Schriver CR, Beaudet AL, Sly WS, Valle D (eds). The Metabolic and Molecular Bases of Inherited Disease. 8th edn. New York; McGraw-Hill; 2001, pp. 3695–3724.
Peters C . Hematopoietic cell transplantation for storage diseases. In: Forman S, Blume K, Appelbaum F (eds). Thomas' Hematopoietic Cell Transplantation. Blackwell Scientific: London, 2003.
Fasth A, Oskarsdottir S, Tulinius M et al. Bone marrow transplantation in metachromatic leukodystrophy (MLD): disease progress in a boy despite transplantation two years before expected onset of symptoms. In: Ringden O, Hobbs JR, Stewards C (eds). Correction of Genetic Diseases by Transplantation IV. The COGENT Press: Middlesex, 1997, pp. 24–27.
Peters C, Waye JS, Vellodi A . Hematopoietic stem cell transplantation for metachromatic leukodystrophy prior to onset of clinical signs and symptoms. In: Ringden O, Hobbs JR, Stewards C (eds). Correction of Genetic Diseases by Transplantation IV. The COGENT Press: Middlesex, 1997, pp. 34–48.
Solders G, Celsing G, Hagenfeldt L . Bone marrow transplantation for adult metachromatic leukodystrophy. In: Ringden O, Hobbs JR, Stewards C (eds). Correction of Genetic Diseases by Transplantation IV. The COGENT Press: Middlesex, 1997, pp. 32–33.
Malm G, Ringden O, Winiarski J et al. Clinical outcome in four children with metachromatic leukodystrophy treated by bone marrow transplantation. Bone Marrow Transplant 1996; 17: 1003–1008.
Krivit W, Shapiro E, Kennedy W et al. Treatment of late infantile metachromatic leukodystrophy by bone marrow transplantation. N Engl J Med 1990; 322: 28–32.
Koc ON, Peters C, Aubourg P et al. Bone marrow-derived mesenchymal stem cells remain host-derived despite successful hematopoietic engraftment after allogeneic transplantation in patients with lysosomal and peroxisomal storage diseases. Exp Hematol 1999; 27: 1675–1681.
Seitelberger F . Pelizaeus-Merzbacher disease. In: Vinken PJ, Bruyn GW (eds). Handbook of Clinical Neurology, Leucodystrophies and Poliodystrophies. North-Holland: Amsterdam, 1970, p 150.
Wilson GN, Holmes RG, Custer J et al. Zellweger syndrome: diagnostic assays, syndrome delineation, and potential therapy. Am J Med Genet 1986; 24: 69–82.
Goldfischer S, Moore CL, Johnson AB et al. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science 1973; 182: 62–64.
Leegwater PA, Vermeulen G, Konst AA et al. Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat Genet 2001; 29: 383–388.
van der Knaap MS, Barth PG, Gabreels FJ et al. A new leukoencephalopathy with vanishing white matter. Neurology 1997; 48: 845–855.
Miano M, Lanino E, Gatti R et al. Four year follow-up of a case of fucosidosis treated with unrelated donor bone marrow transplantation. Bone Marrow Transplant 2001; 27: 747–751.
Krivit W, Peters C, Shapiro EG . Bone marrow transplantation as effective treatment of central nervous system disease in globoid cell leukodystrophy, metachromatic leukodystrophy, adrenoleukodystrophy, mannosidosis, fucosidosis, aspartylglucosaminuria, Hurler, Maroteaux–Lamy, and Sly syndromes, and Gaucher disease type III. Curr Opin Neurol 1999; 12: 167–176.
Vellodi A, Cragg H, Winchester B et al. Allogeneic bone marrow transplantation for fucosidosis. Bone Marrow Transplant 1995; 15: 153–158.
Erikson A, Groth CG, Mansson JE et al. Clinical and biochemical outcome of marrow transplantation for Gaucher disease of the Norrbottnian type. Acta Paediatr Scand 1990; 79: 680–685.
Ringden O, Groth CG, Erikson A et al. Long-term follow-up of the first successful bone marrow transplantation in Gaucher disease. Transplantation 1988; 46: 66–70.
Ringden O, Groth CG, Erikson A et al. Ten years' experience of bone marrow transplantation for Gaucher disease. Transplantation 1995; 59: 864–870.
Svennerholm L, Erikson A, Groth CG et al. Norrbottnian type of Gaucher disease – clinical, biochemical and molecular biology aspects: successful treatment with bone marrow transplantation. Dev Neurosci 1991; 13: 345–351.
Tsai P, Lipton JM, Sahdev I et al. Allogenic bone marrow transplantation in severe Gaucher disease. Pediatr Res 1992; 31: 503–507.
Rappeport JM, Ginns EI . Bone-marrow transplantation in severe Gaucher's disease. N Engl J Med 1984; 311: 84–88.
Hobbs JR, Jones KH, Shaw PJ et al. Beneficial effect of pre-transplant splenectomy on displacement bone marrow transplantation for Gaucher's syndrome. Lancet 1987; 1: 1111–1115.
Starer F, Sargent JD, Hobbs JR . Regression of the radiological changes of Gaucher's disease following bone marrow transplantation. Br J Radiol 1987; 60: 1189–1195.
Barton NW, Brady RO, Dambrosia JM et al. Replacement therapy for inherited enzyme deficiency--macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med 1991; 324: 1464–1470.
Figueroa ML, Rosenbloom BE, Kay AC et al. A less costly regimen of alglucerase to treat Gaucher's disease. N Engl J Med 1992; 327: 1632–1636.
Schiffmann R, Heyes MP, Aerts JM et al. Prospective study of neurological responses to treatment with macrophage-targeted glucocerebrosidase in patients with type 3 Gaucher's disease. Ann Neurol 1997; 42: 613–621.
Brady RO . Gaucher disease. In: Moser HW (ed.). Handbook of Clinical Neurology: Neurodystrophies and Neurolipidoses. Elsevier Science: Amsterdam, 1996, pp. 123–132.
Wall DA, Grange DK, Goulding P et al. Bone marrow transplantation for the treatment of alpha-mannosidosis. J Pediatr 1998; 133: 282–285.
Autti T, Santavuori P, Raininko R et al. Bone marrow transplantation in aspartylglucosaminuria: MRI of brain suggests normalizing myelination. In: Ringden O, Hobbs JR, Stewards C (eds). Correction of Genetic Diseases by Transplantation IV. The COGENT Press: Middlesex, 1997, p. 92.
Arvio M, Sauna-Aho O, Peippo M . Bone marrow transplantation for aspartylglucosaminuria: follow-up study of transplanted and non-transplanted patients. J Pediatr 2001; 138: 288–290.
Santavuori P, Lauronen L, Kirveskari E et al. Neuronal ceroid lipofuscinoses in childhood. Neurol Sci 2000; 21: S35–S41.
Lake BD, Steward CG, Oakhill A et al. Bone marrow transplantation in late infantile Batten disease and juvenile Batten disease. Neuropediatrics 1997; 28: 80–81.
Lipman RD, Donohue LR, Hoppe P et al. Evidence that lysosomal storage of proteolipids is a cell autonomous process in the motor neuron degeneration (mnd) mouse, a model of neuronal ceroid lipofuscinosis. Neurosci Lett 1996; 219: 111–114.
Westlake VJ, Jolly RD, Jones BR et al. Hematopoietic cell transplantation in fetal lambs with ceroid-lipofuscinosis. Am J Med Genet 1995; 57: 365–368.
Deeg HJ, Shulman HM, Albrechtsen D et al. Batten's disease: failure of allogeneic bone marrow transplantation to arrest disease progression in a canine model. Clin Genet 1990; 37: 264–270.
Krivit W, Whitley CB, Chang PN . Lysosomal storage diseases treated by bone marrow transplantation: Review of 21 patients. In: Johnson FL, Pochedly C (eds). Bone Marrow Transplantation in Children. Raven Press: New York, 1990. pp. 261–287.
Bayever E, August CS, Kamani N et al. Allogeneic bone marrow transplantation for Niemann–Pick disease (type IA). Bone Marrow Transplant 1992; 10: 85–86.
Vellodi A, Hobbs JR, O'Donnell NM et al. Treatment of Niemann-Pick disease type B by allogeneic bone marrow transplantation. Br Med J (Clin Res Ed) 1987; 295: 1375–1376.
Hsu YS, Hwu WL, Huang SF et al. Niemann–Pick disease type C (a cellular cholesterol lipidosis) treated by bone marrow transplantation. Bone Marrow Transplant 1999; 24: 103–107.
Kornfeld S, Sly WS . I-cell disease and psedo-Hurler polydystrophy: disorders of lysosomal enzyme phosphorylation and localization. In: Schriver CR, Beaudet AL, Sly WS, Valle D (eds). The Metabolic and Molecular Bases of Inherited Disease, 8th edn. McGraw-Hill: New-York, 2001, pp. 3469–3482.
Kurobane I, Inoue S, Gotoh Y et al. Biochemical improvement after treatment by bone marrow transplantation in I-cell disease. Tohoku J Exp Med 1986; 150: 63–68.
Yamaguchi K, Hayasaka S, Hara S et al. Improvement of tear lysosomal enzyme levels after treatment with bone marrow transplantation in a patient with I-cell disease. Ophthalmic Res 1989; 21: 226–229.
Grewal SS, Orchard PJ, Krivit W et al. Hematopoietic cell transplantation in I-cell disease. Third Scientific Lysosomal Storage Disorders Congress and Seventh International Symposium on Mucopolysaccharide and Related Diseases, 2002.
Gravel RA, Kaback MM, Proia RL et al. The GM2 gangliosidoses. In: Schriver CR, Beaudet AL, Valle D, Sly WS (eds). The Metabolic and Molecular Bases of Inherited Disease, 8th edn. McGraw-Hill: New York, 2001, pp. 3827–3876.
Suzuki Y, Oshima A, Nanba E . b-Galactosidase deficiency (b-galactosidosis): GM1 gangliosidosis and marquio B disease. In: Schriver CR, Beaudet AL, Valle D, Sly WS (eds). The Metabolic and Molecular Bases of Inherited Disease, 8th edn. McGraw-Hill: New-York, 2001, pp. 3775–3810.
O'Brien JS, Storb R, Raff RF et al. Bone marrow transplantation in canine GM1 gangliosidosis. Clin Genet 1990; 38: 274–280.
Coccia PF . Cells that resorb bone. N Engl J Med 1984; 310: 456–458.
Popoff SN, Marks Jr SC . The heterogeneity of the osteopetroses reflects the diversity of cellular influences during skeletal development. Bone 1995; 17: 437–445.
Felix R, Hofstetter W, Cecchini MG . Recent developments in the understanding of the pathophysiology of osteopetrosis. Eur J Endocrinol 1996; 134: 143–156.
Seifert MF, Popoff SN, Jackson ME et al. Experimental studies of osteopetrosis in laboratory animals. Clin Orthop 1993; 23–33.
Kornak U, Kasper D, Bosl MR et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 2001; 104: 205–215.
Kornak U, Schulz A, Friedrich W et al. Mutations in the a3 subunit of the vacuolar H(+)-ATPase cause infantile malignant osteopetrosis. Hum Mol Genet 2000; 9: 2059–2063.
Frattini A, Orchard PJ, Sobacchi C et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet 2000; 25: 343–346.
Sly WS, Whyte MP, Sundaram V et al. Carbonic anhydrase II deficiency in 12 families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. N Engl J Med 1985; 313: 139–145.
Gerritsen EJ, Vossen JM, van Loo IH et al. Autosomal recessive osteopetrosis: variability of findings at diagnosis and during the natural course. Pediatrics 1994; 93: 247–253.
Jagadha V, Halliday WC, Becker LE et al. The association of infantile osteopetrosis and neuronal storage disease in two brothers. Acta Neuropathol (Berl) 1988; 75: 233–240.
Gerritsen EJ, Vossen JM, Fasth A et al. Bone marrow transplantation for autosomal recessive osteopetrosis. A report from the Working Party on Inborn Errors of the European Bone Marrow Transplantation Group. J Pediatr 1994; 125: 896–902.
McMahon C, Will A, Hu P et al. Bone marrow transplantation corrects osteopetrosis in the carbonic anhydrase II deficiency syndrome. Blood 2001; 97: 1947–1950.
Srinivasan M, Abinun M, Cant AJ et al. Malignant infantile osteopetrosis presenting with neonatal hypocalcaemia. Arch Dis Child Fetal Neonatal Ed 2000; 83: F21–F23.
Eapen M, Davies SM, Ramsay NK et al. Hematopoietic stem cell transplantation for infantile osteopetrosis. Bone Marrow Transplant 1998; 22: 941–946.
Sieff CA, Chessells JM, Levinsky RJ et al. Allogeneic bone-marrow transplantation in infantile malignant osteopetrosis. Lancet 1983; 1: 437–441.
Fischer A, Griscelli C, Friedrich W et al. Bone-marrow transplantation for immunodeficiencies and osteopetrosis: European survey, 1968–1985. Lancet 1986; 2: 1080–1084.
Coccia PF, Krivit W, Cervenka J et al. Successful bone-marrow transplantation for infantile malignant osteopetrosis. N Engl J Med 1980; 302: 701–708.
Cheow HK, Steward CG, Grier DJ . Imaging of malignant infantile osteopetrosis before and after bone marrow transplantation. Pediatr Radiol 2001; 31: 869–875.
Schulz AS, Classen CF, Mihatsch WA et al. HLA-haploidentical blood progenitor cell transplantation in osteopetrosis. Blood 2002; 99: 3458–3460.
Krivit W, Freese D, Chan KW et al. Wolman's disease: a review of treatment with bone marrow transplantation and considerations for the future. Bone Marrow Transplant 1992; 10 (Suppl 1): 97–101.
Krivit W, Peters C, Dusenbery K et al. Wolman disease successfully treated by bone marrow transplantation. Bone Marrow Transplant 2000; 26: 567–570.
Peters C, Khanna W, Krivit W et al. A retrospective study of the factors affecting the developments of carpal tunnel syndrome (CTS) symptoms in Hurler patients after bone marrow transplant. Third Scientific Lysosomal Storage Disorders Congress and seventh International Symposium on Mucopolysaccharide and Related Diseases, Paris, 2002.
Peters C, Orchard PJ, Defor TE et al. Hematopoietic cell transplantation for Hurler syndrome: The University of Minnesota experience from 1983 to 2001. Blood 2001; 98: 667 (Abstr. 2797).
Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W . Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-1H). Bone Marrow Transplant 2002; 30: 215–222.
Acknowledgements
The authors acknowledge the pioneering work of Professors John Hobbs of the Westminster Children's Hospital and William Krivit of the University of Minnesota and the assistance of Drs Paul Orchard and Satkiran Grewal in the preparation of this mini review. The authors also recognize the Working Party on Inborn Errors of the European Bone Marrow Transplant Group, National Marrow Donor Program, International Bone Marrow Transplant Registry, International Storage Disease Collaborative Study Group, and the Correction of Genetic Diseases by Transplantation (COGENT) Society for their roles in promoting international dialogue, collaboration, and cooperation.
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Peters, C., Steward, C. & on behalf of the NMDP, IBMTR, and the Working Party on Inborn Errors of the EBMT. Hematopoietic cell transplantation for inherited metabolic diseases: an overview of outcomes and practice guidelines. Bone Marrow Transplant 31, 229–239 (2003). https://doi.org/10.1038/sj.bmt.1703839
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703839
Keywords
This article is cited by
-
Mafosfamide, a cyclophosphamide analog, causes a proinflammatory response and increased permeability on endothelial cells in vitro
Bone Marrow Transplantation (2023)
-
Genotype–phenotype correlation and natural history analyses in a Chinese cohort with pelizaeus–merzbacher disease
Orphanet Journal of Rare Diseases (2022)
-
Understanding caregiver descriptions of initial signs and symptoms to improve diagnosis of metachromatic leukodystrophy
Orphanet Journal of Rare Diseases (2022)
-
Allogeneic hematopoietic stem cell transplantation for inherited metabolic disorders
International Journal of Hematology (2022)
-
Early clinical course after hematopoietic stem cell transplantation in children with juvenile metachromatic leukodystrophy
Molecular and Cellular Pediatrics (2020)