Abstract
Many tumors evolve sophisticated strategies to evade the immune system, and these represent major obstacles for efficient antitumor immune responses. Here we explored a molecular mechanism of metabolic communication deployed by highly glycolytic tumors for immunoevasion. In contrast to colon adenocarcinomas, melanomas showed comparatively high glycolytic activity, which resulted in high acidification of the tumor microenvironment. This tumor acidosis induced Gprotein–coupled receptor–dependent expression of the transcriptional repressor ICER in tumor-associated macrophages that led to their functional polarization toward a non-inflammatory phenotype and promoted tumor growth. Collectively, our findings identify a molecular mechanism of metabolic communication between non-lymphoid tissue and the immune system that was exploited by high-glycolytic-rate tumors for evasion of the immune system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Human transcriptome datasets for patients with melanoma and colonadenocarcinoma are available from the TCGA Research Network at http://cancergenome.nih.gov/. Mouse BioSample metadata are available in the BioSample database under accessions SRP157911 and SRP157897. The analyzed data are depicted in Figs. 1a,b,g, 2b,d, 4b,e and 7a and in Supplementary Figs. 1a and 2a,b.
References
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Ho, P.-C. & Liu, P.-S. Metabolic communication in tumors: a new layer of immunoregulation for immune evasion. J. Immunother. Cancer 4, 4 (2016).
Weide, B. et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin. Cancer Res. 22, 5487–5496 (2016).
Petrelli, F. et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol. 54, 961–970 (2015).
Diem, S. et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br. J. Cancer 114, 256–261 (2016).
Zhang, J. et al. Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: a systematic review and meta-analysis. Sci. Rep. 5, 9800 (2015).
Christofk, H. R. et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230–233 (2008).
Colegio, O. R. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Damaghi, M. et al. Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat. Commun. 6, 8752 (2015).
Romero-Garcia, S., Moreno-Altamirano, M. M. B., Prado-Garcia, H. & Sánchez-García, F. J. Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front. Immunol. 7, R502 (2016).
Radu, C. G., Nijagal, A., McLaughlin, J., Wang, L. & Witte, O. N. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc. Natl Acad. Sci. USA 102, 1632–1637 (2005).
Justus, C. R., Dong, L. & Yang, L. V. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol. 4, 354 (2013).
Gerweck, L. E. & Seetharaman, K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 56, 1194–1198 (1996).
Carmona-Fontaine, C. et al. Metabolic origins of spatial organization in the tumor microenvironment. Proc. Natl Acad. Sci. USA 114, 2934–2939 (2017).
Qian, B.-Z. & Pollard, J. W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 (2010).
Kojima, N. et al. Inducible cAMP early repressor acts as a negative regulator for kindling epileptogenesis and long-term fear memory. J. Neurosci. 28, 6459–6472 (2008).
Brand, A. et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 24, 657–671 (2016).
Mead, J. R., Hughes, T. R., Irvine, S. A., Singh, N. N. & Ramji, D. P. Interferon-γ stimulates the expression of the inducible cAMP early repressor in macrophages through the activation of casein kinase 2. A potentially novel pathway for interferon-γ-mediated inhibition of gene transcription. J. Biol. Chem. 278, 17741–17751 (2003).
Harzenetter, M. D. et al. Negative regulation of TLR responses by the neuropeptide CGRP is mediated by the transcriptional repressor ICER. J. Immunol. 179, 607–615 (2007).
Kratochvill, F. et al. TNF counterbalances the emergence of M2 tumor macrophages. Cell Rep. 12, 1902–1914 (2015).
Pasparakis, M., Alexopoulou, L., Episkopou, V. & Kollias, G. Immune and inflammatory responses in TNFα-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184, 1397–1411 (1996).
Pollard, J. W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 4, 71–78 (2004).
Saccani, A. et al. p50 nuclear factor-κB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 66, 11432–11440 (2006).
Pollard, J. W. Trophic macrophages in development and disease. Nat. Rev. Immunol. 9, 259–270 (2009).
Biswas, S. K. et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-κB and enhanced IRF-3/STAT1 activation). Blood 107, 2112–2122 (2006).
Ojalvo, L. S., King, W., Cox, D. & Pollard, J. W. High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am. J. Pathol. 174, 1048–1064 (2009).
DeNardo, D. G. et al. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16, 91–102 (2009).
Kuang, D.-M. et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 206, 1327–1337 (2009).
Balch, C. M. et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 27, 6199–6206 (2009).
Dietl, K. et al. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J. Immunol. 184, 1200–1209 (2010).
Fischer, K. et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109, 3812–3819 (2007).
Shime, H. et al. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J. Immunol. 180, 7175–7183 (2008).
Husain, Z., Huang, Y., Seth, P. & Sukhatme, V. P. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J. Immunol. 191, 1486–1495 (2013).
Samuvel, D. J., Sundararaj, K. P., Nareika, A., Lopes-Virella, M. F. & Huang, Y. Lactate boosts TLR4 signaling and NF-κB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J. Immunol. 182, 2476–2484 (2009).
Selleri, S. et al. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget 7, 30193–30210 (2016).
Sanderlin, E. J., Justus, C. R., Krewson, E. A. & Yang, L. V. Emerging roles for the pH-sensing G protein-coupled receptors in response to acidotic stress. Cell Health Cytoskelet. 7, 99–109 (2015).
Murakami, N., Yokomizo, T., Okuno, T. & Shimizu, T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J. Biol. Chem. 279, 42484–42491 (2004).
Porta, C. et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor κB. Proc. Natl Acad. Sci. USA 106, 14978–14983 (2009).
Clausen, B. E., Burkhardt, C., Reith, W., Renkawitz, R. & Förster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999).
Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).
Zeh, H. J., Perry-Lalley, D., Dudley, M. E., Rosenberg, S. A. & Yang, J. C. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol. 162, 989–994 (1999).
Oliveros, J. C. VENNY. An interactive tool for comparing lists with Venn diagrams (BioinfoGP, 2007); http://bioinfogp.cnb.csic.es/tools/venny/index.html
Earle, W. R. Production of malignancy in vitro. IV. The mouse fibroblast cultures and changes seen in the living cells. J. Natl Cancer Inst. 4, 165–212 (1943).
Becker, C. et al. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 54, 950–954 (2005).
R Development Core Team. R: a language and environment for statistical computing. (R Foundation for Statistical Computing, 2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome. Biol. 15, 550 (2014).
Ogata, H. et al. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 27, 29–34 (1999).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Acknowledgements
We are grateful to C.J.M. Melief for critical reading of our manuscript. We thank C. Braun, S. Fischer and S. Hesse-Kerolli for expert technical help. We thank M. Diken (Translationale Onkologie, University Medical Center, Johannes Gutenberg University Mainz) for OVA-expressing B16F10 (B16) melanoma cells; and K. Rajalingam (Institute for Immunology, University Medical Center, Johannes Gutenberg University Mainz) for LLC1 cells. This work was supported by Deutsche Forschungsgemeinschaft (DFG) SFB 1292 TP01 (T.Bopp and E.S.), TP13 (H.S. and H.-C.P.) and TP15 (E.v.S.); by DFG grants BO 3306/1-1, SCHM 1014/7-1, SCHM 1014/5-1, SFB 1066 projects B13 (E.S.) and B8 (T.Bopp and C.B.) and TR SFB 156 (E.v.S., H.-C.P. and H.S.); and by the Universitäres Centrum für Tumorerkrankungen and the Forschungszentrum Immuntherapie, core facility Histology, of the University Medical Center, Johannes Gutenberg University Mainz (E.v.S., E.S. and T.Bopp).
Author information
Authors and Affiliations
Contributions
T.Bohn performed and analyzed most experiments. S.R. performed all bioinformatical analyses and together with M.Klein was responsible for next-generation sequencing-based transcriptome analyses. N.L., T.-J.B., P.A.L., J.H. and D.A.-S. helped design and perform some experiments. E.v.S. performed experiments involving human materials and planned and assessed experiments involving immunohistochemistry. N.K. and S.E. generated Crem–/– and Cremfl/fl mice. S.P. performed experiments involving positron-emission tomography. A.B., K.R., J.P. and M.Kreutz generated and provided tumor cell lines. C.L., D.V. and M.H. designed and performed glycolytic Seahorse measurements. V.P., K.G. and B.W. designed and performed the orthotopic MC38 tumor model. J.K. supervised statistical analyses of all experiments. H.-C.P. and S.M. provided anti-CTLA-4 and helped to perform experiments involving immunological-checkpoint blockade. E.S., C.B. and H.S. helped design, analyze and interpret some of the experiments. T.Bopp supervised the project, designed experiments and wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Relative gene expression of cAMP-controlled genes in SKCM and COAD biopsies.
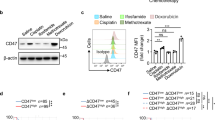
(a) Relative expression of genes that are regulated by cAMP-mediated signaling in primary melanoma (SKCM, n = 104) and colon carcinoma (COAD, n = 285) biopsies. Expression of mRNAs encoding ICER is shown (CREM). (b) Overview of the Crem gene. The green boxes denote exons of Crem and Icer. The red box (Icer-specific exon) highlights the exon that is exclusively part of Icer but not of Crem. ****P ≤ 0.0001, two-tailed, unpaired t test with Welch’s correction.
Supplementary Figure 2 Expression of LDH and ICER positively correlates in human melanoma.
Correlation of LDH and mRNAs encoding ICER in 104 primary human melanoma samples (SCKM – http://cancergenome.nih.gov/). (a) The LDHA and LDHB expression of primary melanomas (n = 104) is divided from the 10th–90th percentile, and expression of mRNAs encoding ICER in LDHA or LDHB high-expressing (blue), LDHA or LDHB low-expressing (orange), and LDHA or LDHB intermediate-expressing (black) primary melanomas is shown. Data are shown as box plots. Center lines represent the median, whiskers indicate minimum and maximum values, boxes indicate the interquartile range. Outliers were identified and removed from the dataset by the ROUT method using GraphPad Prism 7 software. (b) Graph showing expression of the ICER specific exon relative to LDHB expression. Statistical analysis was done using Pearson correlation (r = 0.3485, P = 0.0003). ****P ≤ 0.0001, two-tailed, unpaired t test with Welch’s correction.
Supplementary Figure 3 GPCR132 inhibition leads to reduction of the acid-induced expression of ICER-encoding mRNAs.
Relative expression of mRNAs encoding ICER in BMDMs stimulated under acidic pHe and in the presence of the GPCR132 antagonist l-α-lysophosphatidylcholine (LPC). 1 × 106 BMDMs (n = 4 each condition) were plated in 24-well plate for 8 h (Ctrl.). BMDMs were stimulated under acidic pHe (pH 6.1) by adding the inorganic acid HCl. Additionally, 160 µM or 40 µM of the GPCR132 antagonist LPC was added to the culture. Icer expression was analyzed by qPCR. Individual values are plotted within the bar graph.
Supplementary Figure 4 Cremfl/flLyz2-Cre mouse macrophages show strongly reduced expression of ICER-encoding mRNAs.
Upon RNA isolation of 1 × 106 in vitro–cultured BMDMs (a) or ex vivo–purified TAMs (b) of Cremfl/fl (WT) and Cremfl/flLyz2-Cre mice relative expression of mRNAs encoding ICER was analyzed by qPCR.
Supplementary Figure 5 Low-glycolytic B16 tumors are not rejected by Cremfl/flLyz2-Cre mice.
(a,b), Extracellular acidification rate (ECAR) of (a) indicated tumor cell lines and (b) B16 melanoma sub-lines with high glycolytic activity (B16), low glycolytic activity (Low glycolytic B16) and MC38 colon adenocarcinoma cells (n = 8 each group). The Seahorse assay was repeated four times. Each measurement time point ± s.d. is plotted. (c) Growth of ‘‘Low-glycolytic B16’’ in mice with an ICER-deficiency in myeloid cells (Cremfl/flLyz2-Cre, n = 10) and respective litter-mate control mice (Cremfl/fl, n = 10). 2 × 105 low-glycolytic B16 melanoma cells were inoculated s.c. into the left flank. Tumor development was observed for 21 d. **P ≤ 0.01, ***P ≤ 0.001 using a two-tailed, unpaired t test with Welch’s correction.
Supplementary Figure 6 Ldha/Ldhb double deficiency of B16-Ldhnull melanoma cells.
(a) Western blot analysis of LDHA and LDHB in B16-Ldhnull and WT melanoma cells. 20 µg of total protein extracts of in vitro cultured B16-Ldhnull (Ldhnull) and WT melanoma cells were used for Western Blot analysis. Samples were resolved on a 12% gel and western blotting was performed using anti-LDHA and anti-LDHB antibodies. (b) Lactate concentration in the supernatants of in vitro cultured B16-Ldhnull and WT melanoma cells. ***P ≤ 0.001 using a two-tailed, unpaired t test with Welch’s correction. (c) C57BL/6 WT mice were inoculated with B16-Control or B16-Ldhnull tumors s.c. On day 21 TAM (n = 5 each) were FACS sorted from WT mice bearing tumors and qPCR was conducted to analyze expression of the indicated genes associated with a non-inflammatory macrophage phenotype.
Supplementary Figure 7 Adenylyl cyclase inhibition does not affect B16 proliferation.
Proliferation capacity of MDL-12-treated B16 melanoma cells. 2 × 105 B16 cells were treated with 100 µM MDL-12 for 3 days. As control the solvent was added to the cell culture. After 3 days, 0.5 µCi/well 3H-thymidine was supplemented for 18 h. Uptake of 3H-thymidine was detected using a Β-counter. 6 replicates per group were measured. Data are represented as mean +/− s.d.; data points are plotted as dots.
Supplementary Figure 8 CTLA-4 and MDL-12 administration in B16 bearing B6 mice.
Tumor growth of CTLA-4 antibody– and adenylate cyclase inhibitor MDL-12-treated and untreated (n = 8 per group) B16 bearing C57BL/6 J mice. 2 × 105 B16 melanoma cells were inoculated s.c. into C57BL/6 J mice. On day 6 after tumor cell inoculation, either 0.5 mg CTLA-4 antibody was injected i.v. or additionally 20 µM MDL-12 was injected s.c. peritumoral. The MDL-12 administration was repeated every 3 d. As a control, PBS was injected i.v. and s.c. peritumoral. Tumor size was measured over a time period of 19 d using a caliper. Tumor volume was calculated using the formula width2 × length × 0.5. Individual tumor growth curves are shown.
Supplementary Figure 9 Examples of gating strategy.
Exemplifying gating strategies for FACS analysis and cell sorting are shown. (a) Gating strategy for tumor-associated macrophages. (b) Gating strategy for tumor-infiltrating T cells.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9
Rights and permissions
About this article
Cite this article
Bohn, T., Rapp, S., Luther, N. et al. Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nat Immunol 19, 1319–1329 (2018). https://doi.org/10.1038/s41590-018-0226-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-018-0226-8
This article is cited by
-
Metabolic regulation of tumor-associated macrophage heterogeneity: insights into the tumor microenvironment and immunotherapeutic opportunities
Biomarker Research (2024)
-
MCT4-dependent lactate transport: a novel mechanism for cardiac energy metabolism injury and inflammation in type 2 diabetes mellitus
Cardiovascular Diabetology (2024)
-
What role does GPR65 play in the progression of osteosarcoma? Its mechanism and clinical significance
Cancer Cell International (2024)
-
Carnosine regulation of intracellular pH homeostasis promotes lysosome-dependent tumor immunoevasion
Nature Immunology (2024)
-
The effects of metabolism on the immune microenvironment in colorectal cancer
Cell Death Discovery (2024)