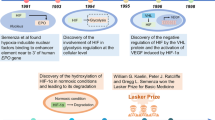
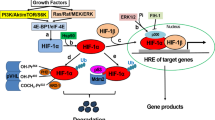
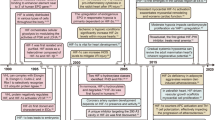
Abstract
Molecular oxygen (O2) sustains intracellular bioenergetics and is consumed by numerous biochemical reactions, making it essential for most species on Earth. Accordingly, decreased oxygen concentration (hypoxia) is a major stressor that generally subverts life of aerobic species and is a prominent feature of pathological states encountered in bacterial infection, inflammation, wounds, cardiovascular defects and cancer. Therefore, key adaptive mechanisms to cope with hypoxia have evolved in mammals. Systemically, these adaptations include increased ventilation, cardiac output, blood vessel growth and circulating red blood cell numbers. On a cellular level, ATP-consuming reactions are suppressed, and metabolism is altered until oxygen homeostasis is restored. A critical question is how mammalian cells sense oxygen levels to coordinate diverse biological outputs during hypoxia. The best-studied mechanism of response to hypoxia involves hypoxia inducible factors (HIFs), which are stabilized by low oxygen availability and control the expression of a multitude of genes, including those involved in cell survival, angiogenesis, glycolysis and invasion/metastasis. Importantly, changes in oxygen can also be sensed via other stress pathways as well as changes in metabolite levels and the generation of reactive oxygen species by mitochondria. Collectively, this leads to cellular adaptations of protein synthesis, energy metabolism, mitochondrial respiration, lipid and carbon metabolism as well as nutrient acquisition. These mechanisms are integral inputs into fine-tuning the responses to hypoxic stress.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pouyssegur, J. & López-Barneo, J. Hypoxia in health and disease. Mol. Asp. Med. 47–48, 1–2 (2016).
Taylor, C. T., Doherty, G., Fallon, P. G. & Cummins, E. P. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J. Clin. Invest. 126, 3716–3724 (2016).
Semenza, G. L. Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012).
Keith, B., Johnson, R. S. & Simon, M. C. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 12, 9–22 (2011).
Kaelin, W. G. et al. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008). Together with Semenza (2012), this review provides an excellent outline of the regulation and importance of HIFs in physiology and diseases.
Markolovic, S., Wilkins, S. E. & Schofield, C. J. Protein hydroxylation catalyzed by 2-oxoglutarate-dependent oxygenases. J. Biol. Chem. 290, 20712–20722 (2015).
Takeda, K. et al. Placental but not heart defects are associated with elevated hypoxia-inducible factor levels in mice lacking prolyl hydroxylase domain protein 2. Mol. Cell. Biol. 26, 8336–8346 (2006).
Lando, D., Peet, D. J., Whelan, D. A., Gorman, J. J. & Whitelaw, M. L. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science 295, 858–861 (2002).
Zhang, N. et al. The asparaginyl hydroxylase factor inhibiting HIF-1α is an essential regulator of metabolism. Cell Metab. 11, 364–378 (2010).
Cockman, M. E., Webb, J. D., Kramer, H. B., Kessler, B. M. & Ratcliffe, P. J. Proteomics-based identification of novel factor inhibiting hypoxia-inducible factor (FIH) substrates indicates widespread asparaginyl hydroxylation of ankyrin repeat domain-containing proteins. Mol. Cell. Proteom. 8, 535–546 (2009).
Cockman, M. E. et al. Lack of activity of recombinant HIF prolyl hydroxylases (PHDs) on reported non-HIF substrates. eLife 8, e46490 (2019).
Lee, F. S. Substrates of PHD. Cell Metab. 30, 626–627 (2019).
Hirsilä, M., Koivunen, P., Günzler, V., Kivirikko, K. I. & Myllyharju, J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J. Biol. Chem. 278, 30772–30780 (2003).
Ast, T. & Mootha, V. K. Oxygen and mammalian cell culture: are we repeating the experiment of Dr. Ox? Nat. Metab. 1, 858–860 (2019).
Koivunen, P., Hirsilä, M., Günzler, V., Kivirikko, K. I. & Myllyharju, J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J. Biol. Chem. 279, 9899–9904 (2004).
Jiang, B. H., Semenza, G. L., Bauer, C. & Marti, H. H. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 271, C1172–C1180 (1996).
Pan, Y. et al. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol. Cell. Biol. 27, 912–925 (2007).
Hagen, T., Taylor, C. T., Lam, F. & Moncada, S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1α. Science 302, 1975–1978 (2003).
Yang, J. et al. Human CHCHD4 mitochondrial proteins regulate cellular oxygen consumption rate and metabolism and provide a critical role in hypoxia signaling and tumor progression. J. Clin. Invest. 122, 600–611 (2012).
Chandel, N. S. et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275, 25130–25138 (2000).
Brunelle, J. K. et al. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 1, 409–414 (2005).
Chandel, N. S. et al. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl Acad. Sci. USA 95, 11715–11720 (1998). This key paper initially links mitochondria to hypoxia-induced gene transcription.
Mansfield, K. D. et al. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-α activation. Cell Metab. 1, 393–399 (2005).
Guzy, R. D. et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 1, 401–408 (2005).
Lin, X. et al. A chemical genomics screen highlights the essential role of mitochondria in HIF-1 regulation. Proc. Natl Acad. Sci. USA 105, 174–179 (2008).
Lee, G. et al. Oxidative dimerization of PHD2 is responsible for its inactivation and contributes to metabolic reprogramming via HIF-1α activation. Sci. Rep. 6, 18928 (2016).
Reczek, C. R. & Chandel, N. S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 33, 8–13 (2015).
Briggs, K. J. et al. Paracrine induction of HIF by glutamate in breast cancer: EglN1 senses cysteine. Cell 166, 126–139 (2016).
King, A., Selak, M. A. & Gottlieb, E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene 25, 4675–4682 (2006).
Selak, M. A. et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7, 77–85 (2005).
Pollard, P. J. et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1α in tumours which result from germline FH and SDH mutations. Hum. Mol. Genet. 14, 2231–2239 (2005). Together with Selak et al. (2005), this paper provides the initial connection of mitochondrial TCA cycle metabolites to regulation of PHDs.
Ryan, D. G. et al. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat. Metab. 1, 16–33 (2019).
Intlekofer, A. M. et al. l-2-Hydroxyglutarate production arises from noncanonical enzyme function at acidic pH. Nat. Chem. Biol. 13, 494–500 (2017).
Nadtochiy, S. M. et al. Acidic pH Is a metabolic switch for 2-hydroxyglutarate generation and signaling. J. Biol. Chem. 291, 20188–20197 (2016).
Sonenberg, N. & Hinnebusch, A. G. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136, 731–745 (2009).
Jackson, R. J., Hellen, C. U. T. & Pestova, T. V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 (2010).
van den Beucken, T. et al. Translational control is a major contributor to hypoxia induced gene expression. Radiother. Oncol. 99, 379–384 (2011).
Feldman, D. E., Chauhan, V. & Koong, A. C. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol. Cancer Res. 3, 597–605 (2005).
Brewer, J. W. & Diehl, J. A. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc. Natl Acad. Sci. USA 97, 12625–12630 (2000).
Blais, J. D. et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol. Cell. Biol. 26, 9517–9532 (2006).
Koumenis, C. et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2α. Mol. Cell. Biol. 22, 7405–7416 (2002).
Arsham, A. M., Howell, J. J. & Simon, M. C. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem. 278, 29655–29660 (2003).
Koritzinsky, M. et al. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 25, 1114–1125 (2006).
Connolly, E., Braunstein, S., Formenti, S. & Schneider, R. J. Hypoxia inhibits protein synthesis through a 4E-BP1 and elongation factor 2 kinase pathway controlled by mTOR and uncoupled in breast cancer cells. Mol. Cell. Biol. 26, 3955–3965 (2006).
Kenney, J. W., Moore, C. E., Wang, X. & Proud, C. G. Eukaryotic elongation factor 2 kinase, an unusual enzyme with multiple roles. Adv. Biol. Regul. 55, 15–27 (2014).
Moore, C. E. J. et al. Elongation factor 2 kinase is regulated by proline hydroxylation and protects cells during hypoxia. Mol. Cell. Biol. 35, 1788–1804 (2015).
Romero-Ruiz, A. et al. Prolyl hydroxylase-dependent modulation of eukaryotic elongation factor 2 activity and protein translation under acute hypoxia. J. Biol. Chem. 287, 9651–9658 (2012).
Feng, T. et al. Optimal translational termination requires C4 lysyl hydroxylation of eRF1. Mol. Cell 53, 645–654 (2014).
Harding, H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Harding, H. P. et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 (2000).
Han, J. et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 15, 481–490 (2013).
Quirós, P. M. et al. Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J. Cell Biol. 216, 2027–2045 (2017).
Kozak, M. A second look at cellular mRNA sequences said to function as internal ribosome entry sites. Nucleic Acids Res. 33, 6593–6602 (2005).
King, H. A., Cobbold, L. C. & Willis, A. E. The role of IRES trans-acting factors in regulating translation initiation. Biochem. Soc. Trans. 38, 1581–1586 (2010).
Stein, I. et al. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol. Cell. Biol. 18, 3112–3119 (1998).
Gan, W. & Rhoads, R. E. Internal initiation of translation directed by the 5′-untranslated region of the mRNA for eIF4G, a factor involved in the picornavirus-induced switch from cap-dependent to internal initiation. J. Biol. Chem. 271, 623–626 (1996).
Young, R. M. et al. Hypoxia-mediated selective mRNA translation by an internal ribosome entry site-independent mechanism. J. Biol. Chem. 283, 16309–16319 (2008).
Lang, K. J. D., Kappel, A. & Goodall, G. J. Hypoxia-inducible factor-1α mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol. Biol. Cell 13, 1792–1801 (2002).
Hinnebusch, A. G., Ivanov, I. P. & Sonenberg, N. Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science 352, 1413–1416 (2016).
Schwerk, J. & Savan, R. Translating the untranslated region. J. Immunol. 195, 2963–2971 (2015).
Ho, J. J. D. et al. Systemic reprogramming of translation efficiencies on oxygen stimulus. Cell Rep. 14, 1293–1300 (2016).
Uniacke, J. et al. An oxygen-regulated switch in the protein synthesis machinery. Nature 486, 126–129 (2012).
Uniacke, J., Kishan Perera, J., Lachance, G., Francisco, C. B. & Lee, S. Cancer cells exploit eIF4E2-directed synthesis of hypoxia response proteins to drive tumor progression. Cancer Res. 74, 1379–1389 (2014). Together with Uniacke et al. (2012), this work demonstrates oxygen-mediated changes to protein synthesis via HIF as a translation initiation factor.
Sørensen, B. S., Busk, M., Overgaard, J., Horsman, M. R. & Alsner, J. Simultaneous hypoxia and low extracellular pH suppress overall metabolic rate and protein synthesis in vitro. PLoS One 10, e0134955 (2015).
Walton, Z. E. et al. Acid suspends the circadian clock in hypoxia through inhibition of mTOR. Cell 174, 72–87 (2018).
Hermesh, O. & Jansen, R.-P. Take the (RN)A-train: localization of mRNA to the endoplasmic reticulum. Biochim. Biophys. Acta Mol. Cell Res. 1833, 2519–2525 (2013).
Staudacher, J. J. et al. Hypoxia-induced gene expression results from selective mRNA partitioning to the endoplasmic reticulum. Nucleic Acids Res. 43, 3219–3236 (2015).
Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 (2012).
Almanza, A. et al. Endoplasmic reticulum stress signalling — from basic mechanisms to clinical applications. FEBS J. 286, 241–278 (2019).
Bouchecareilh, M., Higa, A., Fribourg, S., Moenner, M. & Chevet, E. Peptides derived from the bifunctional kinase/RNase enzyme IRE1α modulate IRE1α activity and protect cells from endoplasmic reticulum stress. FASEB J. 25, 3115–3129 (2011).
Liu, C. Y., Schröder, M. & Kaufman, R. J. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J. Biol. Chem. 275, 24881–24885 (2000).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Lee, K. et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16, 452–466 (2002).
So, J.-S. et al. Silencing of lipid metabolism genes through IRE1α-mediated mRNA decay lowers plasma lipids in mice. Cell Metab. 16, 487–499 (2012).
Xie, H. et al. IRE1α Rnase-dependent lipid homeostasis promotes survival in Myc-transformed cancers. J. Clin. Invest. 128, 1300–1316 (2018).
Zhou, Y. et al. Regulation of glucose homeostasis through a XBP-1–FoxO1 interaction. Nat. Med. 17, 356–365 (2011).
Liu, J. et al. Inflammation improves glucose homeostasis through IKKβ–XBP1s interaction. Cell 167, 1052–1066 (2016).
Liu, Y. et al. Preventing oxidative stress: a new role for XBP1. Cell Death Differ. 16, 847–857 (2009).
Chen, X. et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature 508, 103–107 (2014).
Romero-Ramirez, L. et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 64, 5943–5947 (2004).
Haze, K., Yoshida, H., Yanagi, H., Yura, T. & Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 (1999).
Ye, J. et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 (2000).
Yamamoto, K. et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev. Cell 13, 365–376 (2007).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Wu, J. et al. ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell 13, 351–364 (2007).
Shen, J., Chen, X., Hendershot, L. & Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 3, 99–111 (2002).
Fawcett, T. W., Martindale, J. L., Guyton, K. Z., Hai, T. & Holbrook, N. J. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem. J. 339, 135–141 (1999).
Rutkowski, D. T. et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 4, e374 (2006).
Bi, M. et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 24, 3470–3481 (2005).
Rouschop, K. M. et al. PERK/eIF2α signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc. Natl Acad. Sci. USA 110, 4622–4627 (2013).
Harding, H. P., Zhang, Y., Bertolotti, A., Zeng, H. & Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5, 897–904 (2000).
Wilson, D. F., Rumsey, W. L., Green, T. J. & Vanderkooi, J. M. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J. Biol. Chem. 263, 2712–2718 (1988).
Cooper, C. E. The steady-state kinetics of cytochrome c oxidation by cytochrome oxidase. Biochim. Biophys. Acta 1017, 187–203 (1990).
Wheaton, W. W. & Chandel, N. S. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am. J. Physiol. Physiol. 300, C385–C393 (2011).
Chandel, N. S., Budinger, G. R. & Schumacker, P. T. Molecular oxygen modulates cytochrome c oxidase function. J. Biol. Chem. 271, 18672–18677 (1996).
Fukuda, R. et al. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129, 111–122 (2007).
Hayashi, T. et al. Higd1a is a positive regulator of cytochrome c oxidase. Proc. Natl Acad. Sci. USA 112, 1553–1558 (2015).
Tello, D. et al. Induction of the mitochondrial NDUFA4L2 protein by HIF-1α decreases oxygen consumption by inhibiting complex I activity. Cell Metab. 14, 768–779 (2011).
Chan, S. Y. et al. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron–sulfur cluster assembly proteins ISCU1/2. Cell Metab. 10, 273–284 (2009).
Chen, Z., Li, Y., Zhang, H., Huang, P. & Luthra, R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 29, 4362–4368 (2010).
Puisségur, M.-P. et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 18, 465–478 (2011).
Iyer, N. V. et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 12, 149–162 (1998).
Kim, J. W., Tchernyshyov, I., Semenza, G. L. & Dang, C. V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 (2006).
Papandreou, I., Cairns, R. A., Fontana, L., Lim, A. L. & Denko, N. C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 3, 187–197 (2006). Together with Kim et al. (2006), this work provides initial links between HIF-1 target genes and inhibition of mitochondrial metabolism.
Garcia-Bermudez, J. et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat. Cell Biol. 20, 775–781 (2018).
Ameri, K. et al. HIGD1A regulates oxygen consumption, ROS production, and AMPK activity during glucose deprivation to modulate cell survival and tumor growth. Cell Rep. 10, 891–899 (2015).
Thomas, L. W., Staples, O., Turmaine, M. & Ashcroft, M. CHCHD4 regulates intracellular oxygenation and perinuclear distribution of mitochondria. Front. Oncol. 7, 71 (2017).
Al-Mehdi, A.-B. et al. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal. 5, ra47 (2012).
Fuhrmann, D. C. & Brüne, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 12, 208–215 (2017).
Kim, H. et al. Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia. Mol. Cell 44, 532–544 (2011).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Liu, L. et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 14, 177–185 (2012).
Balaban, R. S., Nemoto, S. & Finkel, T. Mitochondria, oxidants, and aging. Cell 120, 483–495 (2005).
Packer, L. & Fuehr, K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature 267, 423–425 (1977).
Hekimi, S., Lapointe, J. & Wen, Y. Taking a ‘good’ look at free radicals in the aging process. Trends Cell Biol. 21, 569–576 (2011).
Bell, E. L., Klimova, T. A., Eisenbart, J., Schumacker, P. T. & Chandel, N. S. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol. Cell. Biol. 27, 5737–5745 (2007).
Schulz, T. J. et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 6, 280–293 (2007).
Liu, X. et al. Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 19, 2424–2434 (2005).
Bell, E. L. & Chandel, N. S. Mitochondrial oxygen sensing: regulation of hypoxia-inducible factor by mitochondrial generated reactive oxygen species. Essays Biochem. 43, 17–27 (2007).
Lee, S.-J., Hwang, A. B. & Kenyon, C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr. Biol. 20, 2131–2136 (2010).
Weir, E. K., López-Barneo, J., Buckler, K. J. & Archer, S. L. Acute oxygen-sensing mechanisms. N. Engl. J. Med. 353, 2042–2055 (2005).
Sommer, N. et al. Mitochondrial complex IV subunit 4 isoform 2 is essential for acute pulmonary oxygen sensing. Circ. Res. 121, 424–438 (2017).
Moreno-Domínguez, A. et al. Acute O2 sensing through HIF2α-dependent expression of atypical cytochrome oxidase subunits in arterial chemoreceptors. Sci. Signal. 13, eaay9452 (2019).
Fernández-Agüera, M. C. et al. Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metab. 22, 825–837 (2015).
Waypa, G. B. et al. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am. J. Respir. Crit. Care Med. 187, 424–432 (2013). This paper provides genetic evidence that the mitochondrial respiratory chain is necessary for organismal acute responses to hypoxia.
Schleifer, G. et al. Impaired hypoxic pulmonary vasoconstriction in a mouse model of Leigh syndrome. Am. J. Physiol. Cell. Mol. Physiol. 316, L391–L399 (2019).
Chance, B. & Williams, G. R. The respiratory chain and oxidative phosphorylation. Adv. Enzymol. Relat. Subj. Biochem. 17, 65–134 (1956).
Milligan, L. P. & McBride, B. W. Energy costs of ion pumping by animal tissues. J. Nutr. 115, 1374–1382 (1985).
Helenius, I. T., Dada, L. A. & Sznajder, J. I. Role of ubiquitination in Na,K-ATPase regulation during lung injury. Proc. Am. Thorac. Soc. 7, 65–70 (2010).
Emerling, B. M. et al. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free. Radic. Biol. Med. 46, 1386–1391 (2009).
Mungai, P. T. et al. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol. Cell. Biol. 31, 3531–3545 (2011).
Gusarova, G. A. et al. Hypoxia leads to Na,K-ATPase downregulation via Ca2+ release-activated Ca2+ channels and AMPK activation. Mol. Cell. Biol. 31, 3546–3556 (2011).
Laderoute, K. R. et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol. Cell. Biol. 26, 5336–5347 (2006).
Garcia, D. & Shaw, R. J. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 66, 789–800 (2017).
Liu, L. et al. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol. Cell 21, 521–531 (2006).
Orr, A. L. et al. Suppressors of superoxide production from mitochondrial complex III. Nat. Chem. Biol. 11, 834–836 (2015).
Muller, F. L., Liu, Y. & Van Remmen, H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 279, 49064–49073 (2004).
Hernansanz-Agustín, P. et al. Mitochondrial Na+ import controls oxidative phosphorylation and hypoxic redox signalling. bioRxiv https://doi.org/10.1101/385690 (2018).
Szibor, M. et al. Broad AOX expression in a genetically tractable mouse model does not disturb normal physiology. Dis. Model. Mech. 10, 163–171 (2017).
Samanta, D. & Semenza, G. L. Metabolic adaptation of cancer and immune cells mediated by hypoxia-inducible factors. Biochim. Biophys. Acta Rev. Cancer 1870, 15–22 (2018). This timely review article summarizes how HIFs regulate immune cells within the tumour microenvironment.
Nakazawa, M. S., Keith, B. & Simon, M. C. Oxygen availability and metabolic adaptations. Nat. Rev. Cancer 16, 663–673 (2016).
Xie, H. & Simon, M. C. Oxygen availability and metabolic reprogramming in cancer. J. Biol. Chem. 292, 16825–16832 (2017).
Faubert, B. et al. Lactate metabolism in human lung tumors. Cell 171, 358–371 (2017).
Jang, C. et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 30, 594–606.e3 (2019).
Morotti, M. et al. Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in breast cancer. Proc. Natl Acad. Sci. USA 116, 12452–12461 (2019).
Sun, R. C. & Denko, N. C. Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth. Cell Metab. 19, 285–292 (2014). This important paper indicates why hypoxia promotes reductive carboxylation of α-ketoglutarate via isocitrate dehydrogenase by degrading an α-ketoglutarate dehydrogenase complex subunit, and alters use of glutamine carbons for lipid synthesis.
Huang, D. et al. HIF-1-mediated suppression of acyl-CoA dehydrogenases and fatty acid oxidation is critical for cancer progression. Cell Rep. 8, 1930–1942 (2014).
Kamphorst, J. J. et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl Acad. Sci. USA 110, 8882–8887 (2013).
Young, R. M. et al. Dysregulated mTORC1 renders cells critically dependent on desaturated lipids for survival under tumor-like stress. Genes Dev. 27, 1115–1131 (2013). Together with Kamphorst et al. (2013), this paper demonstrates that tumour-relevant oxygen levels can inhibit enzymatic activity of the prominent fatty acyl desaturase SCD1.
Peck, B. & Schulze, A. Lipid desaturation — the next step in targeting lipogenesis in cancer? FEBS J. 283, 2767–2778 (2016).
Shimabukuro, M., Zhou, Y.-T., Levi, M. & Unger, R. H. Fatty acid-induced cell apoptosis: a link between obesity and diabetes. Proc. Natl Acad. Sci. USA 95, 2498–2502 (1998).
Qiu, B. et al. HIF2-dependent lipid storage promotes endoplasmic reticulum homeostasis in clear-cell renal cell carcinoma. Cancer Discov. 5, 652–667 (2015).
Ackerman, D. et al. Triglycerides promote lipid homeostasis during hypoxic stress by balancing fatty acid saturation. Cell Rep. 24, 2596–2605.e5 (2018).
Samanta, D. & Semenza, G. L. Serine synthesis helps hypoxic cancer stem cells regulate redox. Cancer Res. 76, 6458–6462 (2016).
Levine, B. & Klionsky, D. J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 6, 463–477 (2004).
Feng, Y., He, D., Yao, Z. & Klionsky, D. J. The machinery of macroautophagy. Cell Res. 24, 24–41 (2014).
Kimmelman, A. C. & White, E. Autophagy and tumor metabolism. Cell Metab. 25, 1037–1043 (2017).
Mazure, N. M. & Pouysségur, J. Hypoxia-induced autophagy: cell death or cell survival? Curr. Opin. Cell Biol. 22, 177–180 (2010).
Choi, A. M. K., Ryter, S. W. & Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 368, 651–662 (2013).
Poillet-Perez, L. & White, E. Role of tumor and host autophagy in cancer metabolism. Genes Dev. 33, 610–619 (2019).
Zhang, H. et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to Hypoxia. J. Biol. Chem. 283, 10892–10903 (2008).
Bellot, G. et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell. Biol. 29, 2570–2581 (2009).
Pouysségur, J., Dayan, F. & Mazure, N. M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441, 437–443 (2006).
Azad, M. B. et al. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 4, 195–204 (2008).
Fei, P. et al. Bnip3L is induced by p53 under hypoxia, and its knockdown promotes tumor growth. Cancer Cell 6, 597–609 (2004).
Mazure, N. M. & Pouysségur, J. Atypical BH3-domains of BNIP3 and BNIP3L lead to autophagy in hypoxia. Autophagy 5, 868–869 (2009).
Band, M., Joel, A., Hernandez, A. & Avivi, A. Hypoxia-induced BNIP3 expression and mitophagy: in vivo comparison of the rat and the hypoxia-tolerant mole rat, Spalax ehrenbergi. FASEB J. 23, 2327–2335 (2009).
Chourasia, A. H. & Macleod, K. F. Tumor suppressor functions of BNIP3 and mitophagy. Autophagy 11, 1937–1938 (2015).
Rozpedek, W. et al. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr. Mol. Med. 16, 533–544 (2016).
Mihaylova, M. M. & Shaw, R. J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 13, 1016–1023 (2011).
Hardie, D. G. AMPK and autophagy get connected. EMBO J. 30, 634–635 (2011).
Wang, C. et al. Phosphorylation of ULK1 affects autophagosome fusion and links chaperone-mediated autophagy to macroautophagy. Nat. Commun. 9, 3492 (2018).
Russell, R. C. et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 15, 741–750 (2013).
Egan, D. F. et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461 (2011).
Frezza, C. et al. Metabolic profiling of hypoxic cells revealed a catabolic signature required for cell survival. PLoS One 6, e24411 (2011).
Antonescu, C. N., McGraw, T. E. & Klip, A. Reciprocal regulation of endocytosis and metabolism. Cold Spring Harb. Perspect. Biol. 6, a016964 (2014).
Huang, S. & Czech, M. P. The GLUT4 glucose transporter. Cell Metab. 5, 237–252 (2007).
Herman, M. A. & Kahn, B. B. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J. Clin. Invest. 116, 1767–1775 (2006).
Foley, K., Boguslavsky, S. & Klip, A. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry 50, 3048–3061 (2011).
Fazakerley, D. J. et al. Kinetic evidence for unique regulation of GLUT4 trafficking by insulin and AMP-activated protein kinase activators in L6 myotubes. J. Biol. Chem. 285, 1653–1660 (2010).
Mu, J., Brozinick, J. T., Valladares, O., Bucan, M. & Birnbaum, M. J. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell 7, 1085–1094 (2001).
Sakagami, H. et al. Loss of HIF-1α impairs GLUT4 translocation and glucose uptake by the skeletal muscle cells. Am. J. Physiol. Metab. 306, E1065–E1076 (2014).
Görgens, S. W. et al. Hypoxia in combination with muscle contraction improves insulin action and glucose metabolism in human skeletal muscle via the HIF-1α pathway. Diabetes 66, 2800–2807 (2017).
Li, G., Wang, J., Ye, J., Zhang, Y. & Zhang, Y. PPARα protein expression was increased by four weeks of intermittent hypoxic training via ampkα2-dependent manner in mouse skeletal muscle. PLoS One 10, e0122593 (2015).
Siques, P. et al. Long-term chronic intermittent hypobaric hypoxia induces glucose transporter (GLUT4) translocation through AMP-activated protein kinase (AMPK) in the soleus muscle in lean rats. Front. Physiol. 9, 799 (2018).
Wang, Y. et al. Effects of four weeks intermittent hypoxia intervention on glucose homeostasis, insulin sensitivity, GLUT4 translocation, insulin receptor phosphorylation, and Akt activity in skeletal muscle of obese mice with type 2 diabetes. PLoS One 13, e0203551 (2018).
Lee, K. Y., Gesta, S., Boucher, J., Wang, X. L. & Kahn, C. R. The differential role of Hif1β/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation. Cell Metab. 14, 491–503 (2011).
Bloomfield, G. & Kay, R. R. Uses and abuses of macropinocytosis. J. Cell Sci. 129, 2697–2705 (2016).
Recouvreux, M. V. & Commisso, C. Macropinocytosis: a metabolic adaptation to nutrient stress in cancer. Front. Endocrinol. 8, 261 (2017).
Commisso, C. et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 (2013).
Palm, W. et al. The utilization of extracellular proteins as nutrients is suppressed by mTORC1. Cell 162, 259–270 (2015).
Kamphorst, J. J. et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75, 544–553 (2015).
Li, X., Egervari, G., Wang, Y., Berger, S. L. & Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 19, 563–578 (2018).
Chen, W. et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 (2016).
Cho, H. et al. On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models. Nature 539, 107–111 (2016).
Courtney, K. D. et al. HIF-2 complex dissociation, target inhibition, and acquired resistance with PT2385, a first-in-class HIF-2 inhibitor, in patients with clear cell renal cell carcinoma. Clin. Cancer Res. 26, 793–803 (2020).
Maxwell, P. H. & Eckardt, K. U. HIF prolyl hydroxylase inhibitors for the treatment of renal anaemia and beyond. Nat. Rev. Nephrol. 12, 157–168 (2016).
Jain, I. H. et al. Leigh syndrome mouse model can be rescued by interventions that normalize brain hyperoxia, but not HIF activation. Cell Metab. 30, 824–832 (2019).
Ast, T. et al. Hypoxia rescues frataxin loss by restoring iron sulfur cluster biogenesis. Cell 177, 1507–1521 (2019).
Krock, B. L., Skuli, N. & Simon, M. C. Hypoxia-induced angiogenesis: good and evil. Genes Cancer 2, 1117–1133 (2011).
Wong, B. W., Marsch, E., Treps, L., Baes, M. & Carmeliet, P. Endothelial cell metabolism in health and disease: impact of hypoxia. EMBO J. 36, 2187–2203 (2017).
Eales, K. L., Hollinshead, K. E. R. & Tennant, D. A. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 5, e190 (2016).
Höckel, M. et al. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother. Oncol. 26, 45–50 (1993).
Brizel, D. M. et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 56, 941–943 (1996).
Vaupel, P., Kelleher, D. K. & Höckel, M. Oxygenation status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin. Oncol. 28, 29–35 (2001).
Eisinger-Mathason, T. S. K. et al. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 3, 1190–1205 (2013).
Sun, J. D. et al. Selective tumor hypoxia targeting by hypoxia-activated prodrug TH-302 inhibits tumor growth in preclinical models of cancer. Clin. Cancer Res. 18, 758–770 (2012).
Denny, W. A. The role of hypoxia-activated prodrugs in cancer therapy. Lancet Oncol. 1, 25–29 (2000).
Baran, N. & Konopleva, M. Molecular pathways: hypoxia-activated prodrugs in cancer therapy. Clin. Cancer Res. 23, 2382–2390 (2017).
Shmakova, A., Batie, M., Druker, J. & Rocha, S. Chromatin and oxygen sensing in the context of JmjC histone demethylases. Biochem. J. 462, 385–395 (2014).
Chakraborty, A. A. et al. Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science 363, 1217–1222 (2019).
Batie, M. et al. Hypoxia induces rapid changes to histone methylation and reprograms chromatin. Science 363, 1222–1226 (2019).
Masson, N. et al. Conserved N-terminal cysteine dioxygenases transduce responses to hypoxia in animals and plants. Science 365, 65–69 (2019).
Licausi, F. et al. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479, 419–422 (2011).
Gibbs, D. J. et al. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479, 415–418 (2011).
Weits, D. A. et al. Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat. Commun. 5, 3425 (2014).
Brugarolas, J. et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18, 2893–2904 (2004).
Sofer, A., Lei, K., Johannessen, C. M. & Ellisen, L. W. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol. Cell. Biol. 25, 5834–5845 (2005).
Ding, M. et al. The mTOR targets 4E-BP1/2 restrain tumor growth and promote hypoxia tolerance in PTEN-driven prostate cancer. Mol. Cancer Res. 16, 682–695 (2018).
Seong, M., Lee, J. & Kang, H. Hypoxia−induced regulation of mTOR signaling by miR-7 targeting REDD1. J. Cell. Biochem. 120, 4523–4532 (2019).
Bernardi, R. et al. PML inhibits HIF-1α translation and neoangiogenesis through repression of mTOR. Nature 442, 779–785 (2006).
Sancak, Y. et al. The rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 (2008).
Li, Y. et al. Bnip3 mediates the hypoxia-induced inhibition on mammalian target of rapamycin by interacting with Rheb. J. Biol. Chem. 282, 35803–35813 (2007).
Cam, H., Easton, J. B., High, A. & Houghton, P. J. mTORC1 signaling under hypoxic conditions is controlled by ATM-dependent phosphorylation of HIF-1α. Mol. Cell 40, 509–520 (2010).
Brugarolas, J. B., Vazquez, F., Reddy, A., Sellers, W. R. & Kaelin, W. G. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4, 147–158 (2003).
Hudson, C. C. et al. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22, 7004–7014 (2002).
Thomas, G. V. et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat. Med. 12, 122–127 (2006).
Acknowledgements
N.S.C. acknowledges support by R35CA197532 from the National Institutes of Health (NIH). M.S.C. is supported by P01CA104838 and R35CA220483 from the NIH. P.L. is supported by T32AR53461-10 and F32CA217185-02 from the NIH. The authors have no financial interests to disclose. The authors apologize to those authors whose research could not be directly cited due to space limitations.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Molecular Cell Biology thanks C. Taylor, M. Ashcroft and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Dioxygenases
-
A group of enzymes that reduce molecular oxygen by incorporating both oxygen atoms into their substrates.
- von Hippel–Lindau (VHL) tumour suppressor protein
-
(pVHL). A protein named after the physicians von Hippel and Lindau, who characterized patients with highly vascular neoplasia of the kidney, eye and central nervous system who carried mutations in the VHL gene. pVHL is required for the ubiquitylation of hypoxia inducible factor-α and its degradation.
- Carotid body
-
A cluster of peripheral chemoreceptor cells (glomus type I and glomus type II), which sense oxygen, carbon dioxide and pH levels of blood.
- Erythropoietin
-
A glycoprotein cytokine secreted by the kidney in response to hypoxia to stimulate erythropoiesis.
- Elongin BC–CUL2
-
Additional complexes that interact with von Hippel–Lindau (VHL) tumour suppressor protein (pVHL). The Elongin BC complex acts as an adaptor connecting Cullin (Cul) proteins.
- Michaelis constant
-
(Km). The substrate concentration at half of the maximum reaction velocity.
- Acidosis
-
The process or condition where there is increased acidity in the blood and other body tissues.
- Cap-dependent protein synthesis
-
Eukaryotic mRNAs contain a modified guanosine (the cap) at their 5′ ends. Cap-dependent translation requires the binding of an initiation factor, eukaryotic translation initiation factor 4E (eIF4E), to the cap structure.
- PERK
-
(Protein kinase RNA (PKR)-like endoplasmic reticulum (ER) kinase). A sensor of ER stress and stress-induced protein misfolding.
- mTOR
-
(Mechanistic target of rapamycin). A protein kinase that regulates protein synthesis and cell growth in response to growth factors, nutrients, energy levels and stress.
- Eukaryotic initiation factor 2
-
(eIF2). A complex comprising α, β and γ components that integrates a diverse array of stress-related signals to regulate both global and specific mRNA translation.
- ER stress
-
A condition when the capacity of the endoplasmic reticulum (ER) to fold proteins becomes saturated due to impaired protein glycosylation or disulfide bond formation, or by overexpression of or mutations in proteins entering the secretory pathway.
- eIF4F
-
The cap-binding eukaryotic translation initiation factor 4F (eIF4F) complex consists of three subunits, eIF4A, eIF4E and eIF4G. eIF4G strongly associates with eIF4E, the protein that directly binds the mRNA cap.
- ATF4
-
(Activating transcription factor 4). A cAMP-response element binding protein that belongs to the cAMP response element-binding protein 2 (CREB2) family of transcription factors.
- Integrated stress response
-
An adaptive pathway to restore cellular homeostasis by optimizing the cellular response to stress. Its activity is dependent on the cellular context and the type as well as intensity of the stress stimuli.
- Signal recognition particles
-
Universally conserved ribonucleoproteins that recognize and target specific proteins to the endoplasmic reticulum.
- ER-associated degradation
-
The cellular pathway that targets misfolded proteins of the endoplasmic reticulum (ER) for ubiquitylation and subsequent degradation by the proteasome.
- Electron transport chain
-
(ETC). A series of complexes within the inner mitochondrial membrane that shuttle electrons from NADH and FADH2 to molecular oxygen.
- Enzymatic maximal velocity
-
(Vmax). The reaction rate when an enzyme is fully saturated by substrate.
- Iron–sulphur (Fe–S) clusters
-
Molecular ensembles of iron and sulfide, often found as components of electron transfer proteins. The ferredoxin proteins are the most common iron–sulfide clusters in nature.
- Pyruvate
-
The conjugate base, CH3COCOO−, of pyruvic acid is a key intermediate in several metabolic pathways throughout the cell.
- Superoxide dismutases
-
A family of enzymes that catalyse the dismutation of the superoxide (O2−) radical into molecular oxygen or hydrogen peroxide.
- Telomerase
-
A ribonucleoprotein that adds a species-dependent telomere repeat sequence to the 3′ end of a region of repetitive sequences at each end of chromosomes (telomeres).
- Essential electrochemical gradient
-
A gradient of electrochemical potential, usually for an ion that can move across membranes.
- Ubisemiquinone
-
Ubiquinol is the reduced form of coenzyme Q10 that is oxidized by mitochondrial complex III to the partially reduced form ubisemiquinone and subsequently to the fully oxidized ubiquinone. Ubisemiquionone is a highly unstable free radical that can donate electrons to molecular oxygen to generate superoxide.
- Lipid droplets
-
Lipid-rich storage organelles that regulate the storage and hydrolysis of neutral lipids. They also serve as a reservoir for cholesterol and acyl-glycerols for membrane formation and maintenance.
- Pentose phosphate pathway
-
A predominantly anabolic pathway parallel to glycolysis that generates NADPH and precursors for nucleotide synthesis.
- Leigh syndrome
-
A neurological disorder characterized by progressive loss of mental and motor abilities.
Rights and permissions
About this article
Cite this article
Lee, P., Chandel, N.S. & Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol 21, 268–283 (2020). https://doi.org/10.1038/s41580-020-0227-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-020-0227-y
This article is cited by
-
Unraveling the role of HIF-1α in sepsis: from pathophysiology to potential therapeutics—a narrative review
Critical Care (2024)
-
Targeting hypoxia-inducible factors: therapeutic opportunities and challenges
Nature Reviews Drug Discovery (2024)
-
Lactylation constrains OXPHOS under hypoxia
Cell Research (2024)
-
Hypoxia and low-glucose environments co-induced HGDILnc1 promote glycolysis and angiogenesis
Cell Death Discovery (2024)
-
Metabolic regulation of skeletal cell fate and function
Nature Reviews Endocrinology (2024)