Abstract
Background
Supplemental oxygen exposure administered to premature infants is associated with chronic lung disease and abnormal pulmonary function. This study used mild (40%), moderate (60%), and severe (80%) oxygen to determine how hyperoxia-induced changes in lung structure impact pulmonary mechanics in mice.
Methods
C57BL/6J mice were exposed to room air or hyperoxia from birth through postnatal day 8. Baseline pulmonary function and methacholine challenge was assessed at 4 and 8 weeks of age, accompanied by immunohistochemical assessments of both airway (smooth muscle, tethering) and alveolar (simplification, elastin deposition) structure.
Results
Mild/moderate hyperoxia increased baseline airway resistance (40% only) and airway hyperreactivity (40 and 60%) at 4 weeks accompanied by increased airway smooth muscle deposition, which resolved at 8 weeks. Severe hyperoxia increased baseline compliance, baseline resistance, and total elastin/surface area ratio without increasing airway hyperreactivity, and was accompanied by increased alveolar simplification, decreased airway tethering, and changes in elastin distribution at both time points.
Conclusions
Mild to moderate hyperoxia causes changes in airway function and airway hyperreactivity with minimal parenchymal response. Severe hyperoxia drives its functional changes through alveolar simplification, airway tethering, and elastin redistribution. These differential responses can be leveraged to further develop hyperoxia mouse models.
Similar content being viewed by others
Introduction
Bronchopulmonary dysplasia (BPD) is the major pulmonary morbidity of prematurity, affecting up to ten-thousand US infants annually.1 The increasing survival of preterm infants born at lower gestational ages coupled with less invasive ventilatory strategies have changed the pathologic findings associated with BPD from alveolar fibrosis, thickened alveolar septa, and smooth muscle hyperplasia, to alveolar simplification and capillary pruning with less fibrotic changes.2 The pathogenicity of BPD is mediated by several early-life exposures including neonatal oxygen injury, inflammation, and mechanical ventilation,3,4 but their contribution to structural abnormalities in the developing airway and lung parenchyma and impact on pulmonary mechanics is not well understood. Infants with BPD experience functional deficits manifesting as childhood wheezing disorders, increased airway hyperreactivity, and early evidence of obstructive lung disease that persists into adolescence and adulthood.5,6,7,8,9,10 Higher neonatal oxygen exposure predicts BPD diagnoses and further correlates with airway dysfunction among infants without BPD in a dose-dependent manner.4,11 Therefore, there remains a need to understand how hyperoxia-induced structural changes relate to pulmonary function, allowing for a more translational approach and enhanced understanding of pathologic mechanisms of prematurity-related chronic lung disease.
Several animal models of neonatal hyperoxia have attempted to recapitulate the structural features and functional deficits of oxygen exposure on the developing lung.12,13 These models utilized oxygen concentrations ranging from mild (40% O2) to severe (>95% O2) hyperoxia, spanned several developmental lung stages, and performed functional analyses (respiratory mechanics, alveolar diffusion capacity) at different time points.12,14 Each protocol was designed to model specific phenotypic features (alveolar simplification, airway dysfunction) of BPD, but the heterogeneity of hyperoxia protocols (dose, duration, and developmental window) leaves the impression that there are different hyperoxia-induced physiological phenotypes in the airway and parenchyma depending on the exposure paradigm. For example, previous assessments of pulmonary mechanics in hyperoxia showed a minimal increase in baseline airway resistance in mild hyperoxia (40% O2 for 7 days) with conflicting data at higher doses.15,16 Airway hyperreactivity, commonly measured by increased methacholine response, was highest in mild hyperoxia (40% O2 for 7 days) yet blunted with moderate-to-severe hyperoxia (70% O2 for 7 days) in juvenile (3-week-old) mice.16 Conversely, models of severe hyperoxia (100% O2 for 4 days) describe decreased baseline resistance, increased compliance, and only mildly increased sensitivity to methacholine.17 Furthermore, hyperoxia-induced changes in alveolar architecture are most common in severe hyperoxia models with a direct relationship between hyperoxia severity, degree of alveolar simplification, and lung compliance.15,18 Collectively, these studies evaluated different functional outcomes at varying time points, each using their own protocol, and performed limited structural assessments, leaving some ambiguity about mechanical outcomes in a range of hyperoxia models.
The purpose of this study was to perform hyperoxia exposures at increasing doses (40–80% for 8 days) and measure functional (baseline airway mechanics and airway hyperreactivity) and structural (alveolar and airway) changes in adolescent (4-week-old) and adult (8-week-old) mice. We chose an exposure model that spans the saccular and early alveolar stage of murine lung development and allowed for room-air recovery because airway hyperreactivity manifests long after exposure to hyperoxia in former preterm infants. Our aim was to assess the prevalence or distribution of alveolar and airway structures, determine the perturbations of these structures as they relate to hyperoxia, and tie them to changes in pulmonary mechanics. We hypothesized that mild hyperoxia (40%) would cause increased airway resistance and hyperreactivity correlated with changes in airway smooth muscle (ASM), whereas severe hyperoxia (80%) would increase lung compliance mediated through alveolar simplification.
Methods
All protocols were approved by the Institutional Animal Care and Use Committee of University of Rochester (Rochester, NY) and were consistent with The Association for Assessment and Accreditation of Laboratory Animal Care International policies (Frederick, MD).
Animal model
C57BL/6J mouse pups (Jackson Laboratory, Bar Harbor, ME) were monitored until birth then placed into chambers with varying levels of hyperoxia (40, 60, or 80%) or room air control (RA) from within 12 h birth until postnatal day 8. Animals were housed in a specialized inhalation facility on 12-h light−dark cycles with ad libitum standard food and water. Temperature and humidity were monitored throughout the exposure. Animals were recovered in RA until their respective analysis endpoints. Nursing dams were rotated every 24 h.
Pulmonary function testing
Respiratory function testing was performed at 4 weeks and 8 weeks of age for all groups (N = 8–14 per group). Animals were anesthetized with an intraperitoneal ketamine/xylazine mixture (100 mg/kg and 20 mg/kg, respectively), underwent tracheostomy with a blunt-tip metal cannula, and placed on a computer-controlled small animal ventilator (SCIREQ Inc., Montreal, Canada) with a tidal volume of 10 ml/kg, 150 breaths/min, PEEP of 3 cm H2O, and FIO2 of 21%. Once on the ventilator, intraperitoneal pancuronium bromide (10 mg/kg) was given for muscle relaxation and to ensure passive ventilation. Animals were placed on an external heating pad with temperature and heart rate monitoring through the entire protocol. Readouts of positive end expiratory pressure and positive inspiratory pressure were recorded in real -time.
Baseline data and step-wise pressure-volume (PV) curves were obtained after approximately 5 min of equilibration without evidence of spontaneous respiratory effort. Subsequently, nebulized (Aeroneb, SCIREQ Inc. Montreal, Canada) phosphate-buffered saline (PBS) followed by increasing doses of acetyl-β-methylcholine (Sigma-Aldrich, St. Louis, MO) were administered at two-fold doses between 3.125 and 100 μg/ml dissolved in PBS. At each methacholine dose, respiratory function data were obtained using the forced oscillation technique19,20,21 and analyzed using the constant phase model.22,23
Tissue analysis
All tissue analyses were performed on non-ventilated lungs. Lungs were fixed inflated at 25 cm H2O with 10% buffered formalin (Fisher Scientific, Hampton, NH). Inflated lungs were embedded in paraffin and sliced into 4-μm sections. Images were captured using a SPOT RT3 Camera with SPOT Imaging Software (v5.2, Diagnostic Instruments, Inc., Sterling Heights, MI) on a Nikon E800 microscope (Nikon Instruments Inc., Melville, NY) using identical settings in the same session. Sections were stained for hematoxylin and Rubens eosin-phloxine (H&E; Biocare Medical, Concord, CA) as previously described.24 Airways were evaluated for broncho-alveolar attachments (tethers) and alveolar spaces for mean linear intercept (MLI) using H&E sections taken at ×20 magnification. Elastin staining was performed using Hart’s Elastin with Tartrazine (FD&C yellow 5, Alfa Aesar, Haverhill, MA) counterstain as previously described.25 Total elastin staining was determined on airways and separately for alveolar spaces while excluding airway structures via computerized image processing software (ImageJ, NIH, Bethesda, MD) with an identical color threshold set across all images and subtracting any area containing blood vessels to isolate only the alveolar space. Total positive lung surface area was similarly determined, using the same color threshold across images on an inverted image. Elastin staining was expressed as a percent positive staining relative to total stained surface area. Collagen staining was performed with Sirius Red, 0.1% in saturated picric acid (Electron Microscopy Sciences, Hatfield, PA) as previously described26 and total airway collagen staining was determined. All images were analyzed by two investigators blinded to the treatment group.
Immunohistochemistry was performed for alpha smooth muscle actin (α-SMA) using previously described methods.27 Briefly, deparaffinized sections underwent antigen retrieval in citrate buffer, were inoculated with primary antibody for mouse anti-human α-SMA (1:500, Sigma-Aldrich), tagged with Texas Red secondary antibody (1:500, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), and mounted with DAPI Fluoromount-G (SouthernBiotech, Birmingham, AL). Airways were photographed (Nikon microscope, SPOT Camera) at ×20 magnification (>5 per animal, N = 4–6 per treatment), then analyzed using image processing software (ImageJ) for the total amount of positive staining expressed as a ratio to the airway luminal area.
Statistical analysis
Based on historical controls, and knowledge of lung compliance being a sensitive marker of alveolar simplification, eight subjects per experimental group are needed to detect a 20% difference in compliance (α = 0.05, β = 0.2) with 80% power. Secondary comparisons between male and female mice were also performed post hoc. For analyses with both sexes, p value < 0.05 was considered statistically significant, but to assess for trends between sexes given the study was not powered specifically to assess sex differences, a less strict p value of 0.10 was considered significant to justify adding further animals to the study. Statistical analyses were performed in GraphPad PRISM (version 8, La Jolla, CA, United States). Baseline respiratory mechanics, PV curves, and staining analyses were compared using analysis of variance (ANOVA) or Students’ t tests to compare to RA where appropriate. Methacholine dose response was analyzed using two-way repeated measures ANOVA, starting with comparison at the maximal response and moving to lower methacholine doses until significance was no longer observed.
Results
Baseline pulmonary function
Pulmonary function tests were conducted on 4- and 8-week-old mice exposed to RA or 40, 60 or 80% oxygen for the first 8 days of life (N = 8–14 per group).
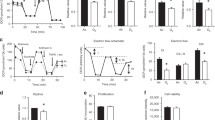
Before analyzing the effects of oxygen, we discovered that lung mechanics were different between 4- and 8-week-old mice under normoxic conditions, reflective of the maturation of the respiratory system. Overall, there were several changes in baseline respiratory function in mice between 4 and 8 weeks of age. Resistance decreased (Fig. 1a, b), compliance increased (Fig. 1c), tissue damping decreased (Fig. 1d), and elastance decreased (Fig. 1e), indicating the maturation of the respiratory system as animals age.
Animals (N = 8–14 per group) were exposed to room air (RA) or different levels of hyperoxia (40, 60, or 80% oxygen) from birth to postnatal day 8 and underwent mechanical ventilation at 4 and 8 weeks of age. a Respiratory system resistance (Rrs) increased in 40% animals and decreased in 80% compared to RA at 4 weeks but normalized at 8 weeks in all groups. b Newtonian Airway resistance (Rn) increased in 40% mice at 4 weeks compared to RA and 80% was increased compared to all groups at 8 weeks. c Static Compliance (Cst) increased in 80% animals at both time points. d Tissue damping (G) decreased in the 80% groups at 4 weeks and remained a trend at 8 weeks. e Tissue elastance (H) decreased in 80% animals at 4 weeks and remained a trend at 8 weeks. f Hysteresivity (η) was largely unchanged across all groups at both time points. Data mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
The effects of neonatal hyperoxia on baseline respiratory mechanics were most prevalent in 4-week-old animals, with attenuation at 8 weeks of age. Total respiratory system resistance (Rrs, Fig. 1a), which includes contribution from the conducting and peripheral airways, the lung tissue, and chest wall, was lower in the 80% group at 4 weeks, and unchanged in all other groups at both time points. Newtonian airway resistance (Rn, Fig. 1b), which isolates the resistance of the conducting airways and is calculated using the constant phase model, however, increased in the 40% group at 4 weeks (with attenuation at 8 weeks), and increased in the 80% group at 8 weeks compared to RA. Baseline static compliance (Cst, Fig. 1c), which signifies the intrinsic elastic properties of the lung and chest wall and is calculated from PV curves, decreased in 40% animals and increased in 80% at 4 weeks of age compared to RA. At 8 weeks, Cst changes in the 40% group normalized, but remained elevated in 80% animals. Tissue damping (G, Fig. 1d), indicating the energy dissipated in the tissues or lost to heat during ventilation, decreased in 80% animals at 4 weeks compared to other groups. Elastance (H, Fig. 1e), or tissue stiffness, decreased in 80% exposed animals at 4 weeks, but also attenuated at 8 weeks. Tissue hysteresivity (η, Fig. 1f), the ratio of tissue damping to elastance and a marker of heterogeneity of ventilation, showed a slight decrease in 40% animals at 4 weeks, but was unchanged at 8 weeks.
Airway hyperreactivity
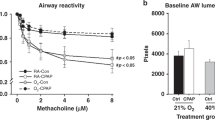
After recording baseline lung mechanics, methacholine, a muscarinic agonist, was administered via nebulization to uncover changes in mechanics related to the presence of ASM (Fig. 2). Increased AHR, defined as increased Rn fold-change from baseline, was observed in all hyperoxia groups at 4 weeks, with magnitude of change at maximum methacholine dose greatest in 40% (8× baseline), followed by 60%, then 80% compared to RA (4× baseline, Fig. 2b). Similar to attenuation in baseline mechanics, AHR normalized at 8 weeks of age among all groups (Fig. 2b). Not surprisingly, airway responses to methacholine had greater magnitude change (5–8× baseline for Rn, Fig. 2b) compared to tissue responses (2–3× baseline for G and H, Fig. 2d, e).
Animals (N = 8–14 per group) were exposed to room air (RA) or different levels of hyperoxia (40, 60, or 80% oxygen) from birth to postnatal day 8 and underwent mechanical ventilation at 4 and 8 weeks of age with a methacholine challenge. a Respiratory system resistance (Rrs) increased in 40% animals at 8 weeks but was unchanged in other groups. b Newtonian airway resistance (Rn) increased in all groups at 4 weeks, with highest magnitude change in 40% animals; all changes resolved at 8 weeks. c Crs did not change with methacholine in any group. d Tissue damping (G) increased in 40% animals at 8 weeks but was unchanged in other groups. e Tissue elastance (H) was unchanged in all groups. f Hysteresivity (η) was largely unchanged in all groups. Data mean ± SEM. *p < 0.05.
Sex differences in baseline mechanics and airway hyperreactivity
Though this study was not powered to specifically determine functional differences between genders, baseline mechanical parameters and AHR were analyzed comparing male and female mice. No differences were observed for any baseline parameters between sexes, and the direction of change after neonatal hyperoxia was similar in all cases (Fig. 3a−f). AHR was assessed among RA and 40% animals (since this group had greatest AHR) with no significant differences or trends between sexes, even when considering a less strict p value (Fig. 3g, h).
Animals (N = 8–14 per group) were exposed to room air (RA) or different levels of hyperoxia (40, 60, or 80%) oxygen from birth to postnatal day 8 and underwent mechanical ventilation at 4 and 8 weeks of age with a methacholine challenge. a−f Baseline mechanics at each time point split by sex. g, h Airway hyperreactivity was assessed by methacholine challenge among the 40% exposed animals because that group demonstrated the greatest airway hyperreactivity. g Respiratory system resistance (Rrs) showed no sex difference in either group. h Newtonian airway resistance showed no sex difference in either group. Data mean ± SEM. *p < 0.05.
Airway structure
Components of airway structure (smooth muscle, elastin, collagen, epithelial thickness, and tethering) were quantified to correlate with changes in baseline airway resistance and airway hyperreactivity. ASM staining was increased in the 40 and 60% groups at 4 weeks and unchanged in 80% treated animals; however, no changes were observed at 8 weeks (Fig. 4a, e). Airway tethering decreased in severe hyperoxia (80%) at both time points (Fig. 4b, f). Finally, airway elastin increased in 60 and 80% animals at 4 weeks, remaining increased in only 80% animals at 8 weeks (Fig. 4d, g) while epithelial thickness (Fig. 4b) and collagen (Fig. 4h, graph not shown) were unchanged.
Room air (RA) and hyperoxia-exposed animals were aged to 4 or 8 weeks of age to assess components of airway remodeling. a, e Ratio of α-smooth muscle actin staining to airway luminal area ratio increased in the 40 and 60% animals at 4 weeks with normalization at 8 weeks. Representative airways from each group showing positive α-smooth muscle actin staining (red), airway lumen (white dotted lines) and outer airway layer (yellow dotted lines). b, c, f H&E sections were assessed for airway epithelial thickness and tethers (arrows). No changes in airway epithelial thickness were observed at both time points. There were significantly less airway tethers present in the 80% group compared to other groups at both time points. d, g Hart’s elastin/Tartazine-stained sections showed increased airway elastin in 80% animals at both time points. h Sirius Red staining for collagen showed no differences in airway deposition at either time point (graph not shown) (representative data mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar = 100 µm.
Alveolar structure
To evaluate hyperoxia-induced changes in alveolar structure, tissue sections were stained with H&E and analyzed to determine mean linear intercept (MLI), a measure that increases with larger alveolar size and alveolar simplification. MLI increased in the 80% group at both time points (Fig. 5a, b). We subsequently stained for elastin to determine changes after hyperoxia exposure. A redistribution of elastin was observed represented by increased elastin to total surface area ratio in the 80% group compared to all other groups at both time points (Fig. 5c, d).
Room air (RA) and hyperoxia-exposed animals underwent H&E and Hart’s elastin/Tartazine staining with quantification in the alveolar space excluding airways. a Mean linear intercept (MLI) increased in 80% lungs at both time points indicating greater alveolar simplification. b Representative H&E tissue sections from all groups showing alveolar simplification in 80% exposed animals. c Elastin staining, expressed as elastin to total surface area ratio increased in the 80% group compared to all other groups at both time points. d Representative sections stained with Hart’s elastin showing elastin redistribution from the secondary septa to areas lining the alveolar surface (arrows). Data mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar = 100 µm.
Discussion
The aim of this study was to determine the effects of different levels (40, 60, and 80% O2) of neonatal hyperoxia for the first 8 postnatal days on the structure and function of the developing lung at two distinct time points. We utilized OxygenAUC, a cumulative level of oxygen exposure that we have used in other clinical and laboratory studies, to model our dose response while being consistent with previous studies in our laboratory that use multiples of 4 days for exposure duration.4,18,28 Additionally, we standardized our exposure duration (PND 0–8) to include a murine developmental window spanning peak secondary alveolar septation, to maximize the opportunity to impact alveolar simplification.18,29,30
In these experiments, we performed several different respiratory function maneuvers to assess the baseline mechanics and response to a methacholine challenge. The normal time course of respiratory system development under normoxic conditions shows altered mechanics with age: Rrs and Rn decreases, Cst increases, G decreases, and H decreases (Fig. 1). Developmentally, mice at 4 weeks approximate to humans in late childhood/early adolescence, when bulk alveolarization is complete31 and childhood wheezing disorders are commonly diagnosed. Adult mice (8 weeks) were evaluated to see if the changes improved or persisted, because long-term follow-up data from former preterm infants are limited and confounded by other factors. This study details how careful analysis of the functional and immunohistochemical structure of the airway and alveolar spaces with knowledge of their known mechanical properties can explain changes in respiratory mechanics secondary to neonatal hyperoxia.
In vivo assessments of pulmonary mechanics require fitting the data from different perturbations to two different mathematical models that characterize the mechanical behavior of the lung and distinguish airway from alveolar responses. Single frequency oscillatory maneuvers measure the resistance, compliance, and elastance of the entire respiratory system, which includes the entire lung and chest wall. Multiple frequency perturbations, however, separate responses of the airway (Rn), and lung parenchyma (G, H, η) using the constant phase model.22 These same principles are applied when a subject is challenged with a bronchoconstrictor (methacholine), revealing hyperresponsiveness that can differ in magnitude between these two lung “compartments”. Finally, pressure−volume maneuvers can assess the distensibility of the respiratory system over the entire inspiratory capacity to characterize Cst when fit to the Salazar−Knowles equation.
Extremely preterm infants have increased airflow obstruction in infancy,32 particularly evident in the middle-to-late forced expiratory flows.33,34 These functional deficits predispose them to AHR and childhood wheezing irrespective of their BPD status6,7,35,36,37,38,39 driving fixed airway disease phenotypically distinct from atopic asthma.40 The study of airway resistance and hyperreactivity is of particular interest in hyperoxia mouse models because neonatal supplemental oxygen exposure correlates with wheezing disorders and use of bronchodilator medications in former preterm infants with and without BPD.4,41 In humans, there is coordinated development of the ASM as it differentiates from the mesenchyme, surrounds the bronchial tree, and extends distally to the terminal lung sac. Functional evidence has shown that ASM hypertrophies in infants born extremely preterm who were treated with supplemental oxygen and mechanical ventilation.42
Term rodents are born in early saccular development, analogous to an extremely preterm infant, allowing for modeling in neonatal hyperoxia models. In our mouse study, mild or moderate hyperoxia (40 and 60% for 8 days) changed airway function with increased baseline Rn (40%), and increased AHR (40 and 60%). These functional changes correlate with increased ASM staining in the 40 and 60% groups. The increased baseline Rn likely represents increased resting ASM tone, making the airway narrower and thus more resistant to flow. When exposed to methacholine, the airway constricts and Rn increases, revealing that AHR is mainly driven by narrowing of the airway caliber. Modeling methacholine responses using the constant phase model revealed a 5–8-fold-change in Rn (Fig. 2b) with only a 2–3-fold difference in G and H (Fig. 2d, e), indicating that AHR was mainly driven by changes in the airway and not tissue mechanics. Our functional findings were consistent with previous studies showing that mild hyperoxia (40% O2 for 7 days) increased ASM proliferation and increased in vivo and in vitro AHR.16,43 Similarly, Kumar et al. showed AHR was increased in aged mice exposed to severe hyperoxia (85% O2 from P3–15) with increased force generated by tracheal rings and increased inflammatory markers.44 Interestingly, we did not find AHR in any 8-week hyperoxia-exposed animals, and found that ASM staining normalized across all groups, though we did not perform isolated functional muscle assessments. Three factors could explain these differences: (1) The dose and duration of O2 exposure was higher in Kumar et al.; (2) the in vivo assessment of increased force occurred at 15 weeks where our ventilation studies ended at 8 weeks; and (3) Our animals are tracheostomized and assessed by forced oscillation, thereby bypassing any upper airway resistance which is still present when calculating resistance by whole body plethysmography. Further studies are required to interrogate the signaling pathways leading to ASM hypertrophy, perhaps through epithelial−mesenchymal cross-talk in the hyperoxia-exposed airway, including why the phenotype resolves in adulthood. The resolution of AHR in the 40 and 60% exposed animals in our study may be consistent with infants “growing out of” their childhood wheezing disorders in adulthood.45 In contrast to moderate hyperoxia, severe hyperoxia increased airway elastin deposition in 80% animals without changes in epithelial thickness or collagen deposition. We speculate that increased airway elastin with similar airway collagen could cause a more distensible airway because of elastin’s linear stress−strain relationship,46 counteracting the resistance from decreased airway tethering to result in similar Rn to RA mice at 4 weeks. Finally, changes in alveolar epithelial thickness have been reported,44 which our analysis and other47 that focused on small airways did not. These discrepancies, again, highlight differences in dose and duration of hyperoxia models and the changes occurring as animals age.
Severe hyperoxia (80% O2) also drives a different functional phenotype in hyperoxia-exposed animals concentrated in the alveolar space. The most striking functional differences in 80% exposed animals were increased Cst, decreased G, and decreased H, indicating a floppy, distensible lung phenotypically consistent with obstructive lung disease. Decreased G (Fig. 3d) correlated closely with alveolar simplification (Fig. 5b), representing less energy lost to heat in the tissues as inspired air travels distally down the broncho-alveolar tree. In addition, there were fewer broncho-alveolar attachments in the 80% exposed animals, which may potentiate airway collapse and explain increases in baseline Rn (Fig. 1b). Our experiments comport with other studies showing proliferation of ASM and fewer bronchiolar-alveolar attachments in aged (10 months) mice after hyperoxia (65% O2 for 14 days),47 though functional outcomes were not part of that study. Furthermore, Nardiello et al. studied alveolar architecture in mild to severe hyperoxia (40–85% for 7 or 14 days) revealing a direct correlation between oxygen dose and alveolar simplification, though 7-day exposures were not performed for the 40 and 60% exposure groups in efforts to model more severe BPD.18 The exposure that most closely matches ours (85% O2 for 7 days) showed decreased MLI, alveolar number, and alveolar density, which is consistent with our 80% group (Fig. 5a, b). It is plausible that if mild to moderate oxygen exposure was extended through the second postnatal week we would have uncovered subtle changes in alveolar development, but they are most striking with more severe hyperoxia. We therefore expect our findings to be similar and speculate that there are two opposing forces influencing respiratory system resistance in severe hyperoxia: (1) Increased airway resistance due to lack of airway tethering and airway collapse and (2) decreased tissue resistance and tissue damping due to alveolar simplification. This explains why Rrs was unchanged in adult mice, despite increased Rn and decreased G. To evaluate compliance changes, elastin staining was performed and quantified in the alveolar space after severe hyperoxia, revealing its geographic redistribution from the secondary septa/tips of budding alveoli to more crescent-shaped, circumferential deposition along the alveolus (Fig. 5c, d, arrows). Elastin is a molecule that provides both resistance and distensibility to the tissues, and likely contributes to increased alveolar compliance. Collagen staining was not different in the alveoli or airway in our models. This highlights the need to better understand extracellular matrix components in hyperoxia/BPD via their abundance, distribution, or post-translational modification, perhaps through the use of mathematical models,48 to understand their contribution to pulmonary mechanics.
One limitation is that this study only used C57BL/6J mice, and mouse strain is an important factor in oxygen sensitivity and airway responses. We chose this strain because it is the most sensitive to hyperoxia-induced perturbations in alveolar development but other strains (BALB/c) are commonly utilized for AHR models, especially in asthma.49,50 We also did not look at other intrapulmonary and extrapulmonary processes that are effected by hyperoxia that we speculate do not relate to pulmonary mechanics. Indeed, other studies show that neonatal hyperoxia exposure decreases alveolar diffusion capacity (another way to assess pulmonary function) and induces capillary rareification and pulmonary hypertension.14 Additionally, there are durable effects of neonatal hyperoxia on body composition, cognitive, cardiac, and renal development51,52,53 which were not evaluated.
The breadth of oxygen exposure protocols in the literature has both positive and negative consequences for investigators studying neonatal oxygen exposures. On one hand, investigators can leverage these differential responses by choosing mild/moderate hyperoxia while studying airway function and severe hyperoxia for alterations in alveolar development. Conversely, the lack of a “gold standard” decreases reproducibility, requiring re-characterization of each paradigm with each different oxygen dose, oxygen duration, and/or rodent strain, among other factors. Several models have been created using neonatal hyperoxia with antenatal (e.g. inflammation), concurrent (e.g. intermittent hypoxia, positive pressure ventilation), or post-exposure (e.g. respiratory viral pathogen) exposures, creating a “double hit” phenomenon that adds another layer of complexity on top of the chosen oxygen protocol. We have established a response to oxygen that transiently drives airway disease (40% for 8 days) independent of the more protracted alveolar disease, which is a different paradigm that several laboratories, including our own, have used in the past. We can now use these models to test if the recovered lung after mild hyperoxia acts the same or differently in response to a “double hit” and interrogate the mechanisms by which lung development is durably altered by neonatal hyperoxia.
References
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Jobe, A. H. The new bronchopulmonary dysplasia. Curr. Opin. Pediatr. 23, 167–172 (2011).
Reyburn, B., Martin, R. J., Prakash, Y. S. & MacFarlane, P. M. Mechanisms of injury to the preterm lung and airway: implications for long-term pulmonary outcome. Neonatology 101, 345–352 (2012).
Stevens, T. P., Dylag, A., Panthagani, I., Pryhuber, G. & Halterman, J. Effect of cumulative oxygen exposure on respiratory symptoms during infancy among VLBW infants without bronchopulmonary dysplasia. Pediatr. Pulmonol. 45, 371–379 (2010).
Balinotti, J. E. et al. Growth of lung parenchyma in infants and toddlers with chronic lung disease of infancy. Am. J. Respir. Crit. Care Med. 181, 1093–1097 (2010).
Been, J. V. et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 11, e1001596 (2014).
Baraldi, E., Carraro, S. & Filippone, M. Bronchopulmonary dysplasia: definitions and long-term respiratory outcome. Early Hum. Dev. 85, S1–S3 (2009).
Brostrom, E. B., Thunqvist, P., Adenfelt, G., Borling, E. & Katz-Salamon, M. Obstructive lung disease in children with mild to severe BPD. Respir. Med. 104, 362–370 (2010).
Doyle, L. W. et al. Ventilation in extremely preterm infants and respiratory function at 8 years. N. Engl. J. Med. 377, 329–337 (2017).
Fawke, J. et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am. J. Respir. Crit. Care Med. 182, 237–245 (2010).
Wai, K. C. et al. Early cumulative supplemental oxygen predicts bronchopulmonary dysplasia in high risk extremely low gestational age newborns. J. Pediatr. 177, 97–102 e2 (2016).
Berger, J. & Bhandari, V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am. J. Physiol. Lung Cell Mol. Physiol. 307, L936–L947 (2014).
Silva, D. M., Nardiello, C., Pozarska, A. & Morty, R. E. Recent advances in the mechanisms of lung alveolarization and the pathogenesis of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 309, L1239–L1272 (2015).
Cox, A. M., Gao, Y., Perl, A. T., Tepper, R. S. & Ahlfeld, S. K. Cumulative effects of neonatal hyperoxia on murine alveolar structure and function. Pediatr. Pulmonol. 52, 616–624 (2017).
Yee, M. et al. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. Am. J. Physiol. Lung Cell Mol. Physiol. 297, L641–L649 (2009).
Wang, H. et al. Severity of neonatal hyperoxia determines structural and functional changes in developing mouse airway. Am. J. Physiol. Lung Cell Mol. Physiol. 307, L295–L301 (2014).
Regal, J. F., Lawrence, B. P., Johnson, A. C., Lojovich, S. J. & O'Reilly, M. A. Neonatal oxygen exposure alters airway hyper-responsiveness but not the response to allergen challenge in adult mice. Pediatr. Allergy Immunol. 25, 180–186 (2014).
Nardiello, C. et al. Standardisation of oxygen exposure in the development of mouse models for bronchopulmonary dysplasia. Dis. Model Mech. 10, 185–196 (2017).
DuBois, A. B., Brody, A. W., Lewis, D. H., Franklin, B. & Burgess, J. Oscillation mechanics of lungs and chest in man. J. Appl. Physiol. 8, 587–594 (1956).
Raffay, T. M. et al. S-Nitrosoglutathione attenuates airway hyperresponsiveness in murine bronchopulmonary dysplasia. Mol. Pharmacol. 90, 418–426 (2016).
Shalaby, K. H., Gold, L. G., Schuessler, T. F., Martin, J. G. & Robichaud, A. Combined forced oscillation and forced expiration measurements in mice for the assessment of airway hyperresponsiveness. Respir. Res. 11, 82 (2010).
Hantos, Z., Daroczy, B., Suki, B., Nagy, S. & Fredberg, J. J. Input impedance and peripheral inhomogeneity of dog lungs. J. Appl. Physiol. (1985) 72, 168–178 (1992).
Schuessler, T. F. & Bates, J. H. A computer-controlled research ventilator for small animals: design and evaluation. IEEE Trans. Bio-Med. Eng. 42, 860–866 (1995).
Feldman, A. T. & Wolfe, D. Tissue processing and hematoxylin and eosin staining. Methods Mol. Biol. (Clifton, NJ) 1180, 31–43 (2014).
Culling, C. F. A. Handbook of Histopathological and Histochemical Techniques Vol. 726 (Butterworth & Co. 1974).
Rittié, L. (ed.). in Fibrosis: Methods and Protocols 395−407 (Springer New York, New York, NY, 2017).
Buczynski, B. W., Yee, M., Martin, K. C., Lawrence, B. P. & O'Reilly, M. A. Neonatal hyperoxia alters the host response to influenza A virus infection in adult mice through multiple pathways. Am. J. Physiol. Lung Cell Mol. Physiol. 305, L282–L290 (2013).
Maduekwe, E. T. et al. Cumulative neonatal oxygen exposure predicts response of adult mice infected with influenza A virus. Pediatr. Pulmonol. 50, 222–230 (2015).
Herriges, M. & Morrisey, E. E. Lung development: orchestrating the generation and regeneration of a complex organ. Development 141, 502–513 (2014).
Hogan, B. L. et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15, 123–138 (2014).
Hislop, A. A., Wigglesworth, J. S. & Desai, R. Alveolar development in the human fetus and infant. Early Hum. Dev. 13, 1–11 (1986).
Hoo, A. F. et al. Impact of ethnicity and extreme prematurity on infant pulmonary function. Pediatr. Pulmonol. 49, 679–687 (2014).
Robin, B. et al. Pulmonary function in bronchopulmonary dysplasia. Pediatr. Pulmonol. 37, 236–242 (2004).
Sanchez-Solis, M. et al. Lung function among infants born preterm, with or without bronchopulmonary dysplasia. Pediatr. Pulmonol. 47, 674–681 (2012).
Kotecha, S. J. et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax 68, 760–766 (2013).
Halvorsen, T. et al. Characteristics of asthma and airway hyper-responsiveness after premature birth. Pediatr. Allergy Immunol. 16, 487–494 (2005).
Colin, A. A., McEvoy, C. & Castile, R. G. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks' gestational age. Pediatrics 126, 115–128 (2010).
Greenough, A. Long-term pulmonary outcome in the preterm infant. Neonatology 93, 324–327 (2008).
Speer, C. P. & Silverman, M. Issues relating to children born prematurely. Eur. Respir. J. Suppl. 27, 13s–16ss (1998).
Filippone, M., Carraro, S. & Baraldi, E. The term “asthma” should be avoided in describing the chronic pulmonary disease of prematurity. Eur. Respir. J. 42, 1430–1431 (2013).
Di Fiore, J. M. et al. Early inspired oxygen and intermittent hypoxemic events in extremely premature infants are associated with asthma medication use at 2 years of age. J. Perinatol. 39, 203–211 (2019).
Sward-Comunelli, S. L., Mabry, S. M., Truog, W. E. & Thibeault, D. W. Airway muscle in preterm infants: changes during development. J. Pediatr. 130, 570–576 (1997).
Belik, J., Jankov, R. P., Pan, J. & Tanswell, A. K. Chronic O2 exposure enhances vascular and airway smooth muscle contraction in the newborn but not adult rat. J. Appl Physiol. (1985) 94, 2303–2312 (2003).
Kumar, V. H. et al. Neonatal hyperoxia increases airway reactivity and inflammation in adult mice. Pediatr. Pulmonol. 51, 1131–1141 (2016).
Wang, A. L., Datta, S., Weiss, S. T. & Tantisira, K. G. Remission of persistent childhood asthma: early predictors of adult outcomes. J. Allergy Clin. Immunol. 143, 1752–9 e6 (2019).
Suki, B., Stamenovic, D. & Hubmayr, R. Lung parenchymal mechanics. Compr. Physiol. 1, 1317–1351 (2011).
O'Reilly, M., Harding, R. & Sozo, F. Altered small airways in aged mice following neonatal exposure to hyperoxic gas. Neonatology 105, 39–45 (2014).
Suki, B. & Bates, J. H. Extracellular matrix mechanics in lung parenchymal diseases. Respir. Physiol. Neurobiol. 163, 33–43 (2008).
Tiono, J. et al. Mouse genetic background impacts susceptibility to hyperoxia-driven perturbations to lung maturation. Pediatr. Pulmonol. 54, 1060–1077 (2019).
Will, J. P. et al. Strain-dependent effects on lung structure, matrix remodeling, and Stat3/Smad2 signaling in C57BL/6N and C57BL/6J mice after neonatal hyperoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 317, R169–R181 (2019).
Kumar, V. H. S. et al. Long-term effects of neonatal hyperoxia in adult mice. Anat. Rec. (Hoboken) 301, 717–726 (2018).
Buczynski, B. W. et al. Lung-specific extracellular superoxide dismutase improves cognition of adult mice exposed to neonatal hyperoxia. Front. Med. (Lausanne) 5, 334 (2018).
Yee, M. et al. Neonatal hyperoxia depletes pulmonary vein cardiomyocytes in adult mice via mitochondrial oxidation. Am. J. Physiol. Lung Cell Mol. Physiol. 314, L846–L859 (2018).
Acknowledgements
The authors would like to acknowledge Robert Gelein for maintaining the oxygen exposure facility, Daria Krenitsky for tissue sectioning and processing, and Ethan David Cohen and Rachel Warren for their technical assistance and advice regarding the manuscript. This study was supported by a grant from the Strong Children’s Research Center at the University of Rochester (A.M.D.) and National Institutes of Health Grants 5R01 HL091968 (M.A.O.). NIH grant P30 ES001247 supported the animal inhalation facility and the tissue-processing core.
Author information
Authors and Affiliations
Contributions
A.M.D., J.H., and M.A.O. were involved in conceptual study design, data acquisition, data analysis, data interpretation, and composing or revising the manuscript. M.Y. was involved in conceptual study design, data acquisition, and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dylag, A.M., Haak, J., Yee, M. et al. Pulmonary mechanics and structural lung development after neonatal hyperoxia in mice. Pediatr Res 87, 1201–1210 (2020). https://doi.org/10.1038/s41390-019-0723-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0723-y
This article is cited by
-
The role of rhIGF-1/BP3 in the prevention of pulmonary hypertension in bronchopulmonary dysplasia and its underlying mechanism
BMC Pulmonary Medicine (2023)
-
Maternal antibiotic exposure disrupts microbiota and exacerbates hyperoxia-induced lung injury in neonatal mice
Pediatric Research (2021)
-
Neonatal hyperoxia enhances age-dependent expression of SARS-CoV-2 receptors in mice
Scientific Reports (2020)