Key Points
-
The innate immune system is crucial for host defence but is also implicated in driving inflammatory diseases of the lower respiratory tract, such as asthma and chronic obstructive pulmonary disease (COPD). This mechanism of pathogenesis is distinct from the conventional view that the adaptive immune system is responsible for chronic inflammatory disease.
-
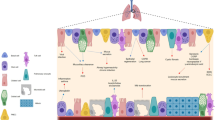
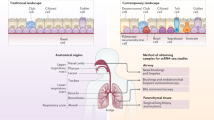
Airway epithelial cells that line the mucosal surface of the lower airways and various immune cell populations (such as natural killer T cells, macrophages and innate lymphoid cells) actively participate in both host defence and disease in the lungs.
-
Airway epithelial cells are equipped with pattern recognition receptors (PRRs) to trigger the innate immune response to microbial pathogens, although the specific subsets of PRRs and the types of airway epithelial cells (for example, ciliated cells, mucous cells, secretory cells and basal cells) that express these receptors still need to be better defined. A key effect of PRR activation is the downstream production of interferons (IFNs) and expression of IFN-stimulated genes, and precisely how this contributes to host defence (particularly against respiratory viruses) is currently under study.
-
Airway epithelial cells are also capable of expansion as a special progenitor or stem cell niche, and this subpopulation of airway progenitor epithelial cells has the capacity for the long-term production of cytokines — for example, interleukin-33 (IL-33) — that can drive type 2 immune responses and consequent airway disease.
-
Innate immune cells can respond to cytokine signals with a type 2 immune response (typified by IL-13 production) that in turn causes remodelling of the airway epithelium to a disease phenotype — for example, mucous cell metaplasia. The precise subsets of innate immune cells that are involved still need to be better defined in humans with chronic airway disease. Similarly, the role of granulocyte populations and the link to non-type 2 cytokine (for example, IL-17) production and consequent airway disease also needs further characterization.
-
New therapeutics aimed at correcting excessive innate immune responses have reached the stage of clinical trials for asthma and COPD. Current trials focus particularly on targeting type 2 cytokines (for example, IL-13) to attenuate chronic airway disease. These approaches are challenged by the need to develop biomarkers that can stratify patients and thereby identify patient subsets that might benefit from specific immunotherapies.
Abstract
An abnormal immune response to environmental agents is generally thought to be responsible for causing chronic respiratory diseases, such as asthma and chronic obstructive pulmonary disease (COPD). Based on studies of experimental models and human subjects, there is increasing evidence that the response of the innate immune system is crucial for the development of this type of airway disease. Airway epithelial cells and innate immune cells represent key components of the pathogenesis of chronic airway disease and are emerging targets for new therapies. In this Review, we summarize the innate immune mechanisms by which airway epithelial cells and innate immune cells regulate the development of chronic respiratory diseases. We also explain how these pathways are being targeted in the clinic to treat patients with these diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Minino, A. M., Murphy, S. L., Xu, J. & Kochanek, K. D. in National vital statistics reports (Centers for Disease Control and Prevention, 2011).
World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. (eds Bousquet, J. & Khaltaev, N.) (WHO, 2007).
Holtzman, M. J. Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens. J. Clin. Invest. 122, 2741–2748 (2012).
Galli, S. J. & Tsai, M. IgE and mast cells in allergic disease. Nature Med. 18, 693–704 (2012).
Spits, H. & Cupedo, T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 30, 647–675 (2012).
Walker, J. A., Barlow, J. L. & McKenzie, A. N. J. Innate lymphoid cells — how did we miss them? Nature Rev. Immunol. 13, 75–87 (2013).
Van Dyken, S. J. & Locksley, R. M. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu. Rev. Immunol. 31, 317–343 (2013).
Siracusa, M. C., Kim, B. S., Spergel, J. M. & Artis, D. Basophils and allergic inflammation. J. Allergy Clin. Immunol. 132, 789–801 (2013).
Paget, C. & Trottein, F. Role of type 1 natural killer T cells in pulmonary immunity. Mucosal Immunol. 6, 1054–1067 (2013).
Holt, P. G., Strickland, D. H., Hales, B. J. & Sly, P. D. Defective respiratory tract immune surveillance in asthma. Chest 145, 370–378 (2014).
Holtzman, M. J. et al. Importance of airway inflammation for hyperresponsiveness induced by ozone. Am. Rev. Respir. Dis. 127, 686–690 (1983).
Boushey, H. A. & Holtzman, M. J. Experimental airway inflammation and hyperresponsiveness: searching for cells and mediators. Am. Rev. Respir. Dis. 131, 312–313 (1985).
Holtzman, M. J. et al. Control of epithelial immune-response genes and implications for airway immunity and inflammation. Proc. Assoc. Am. Phys. 110, 1–11 (1998).
Holgate, S. T. et al. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J. Allergy Clin. Immunol. 105, 193–204 (2000).
Lambrecht, B. & Hammad, H. The airway epithelium in asthma. Nature Med. 18, 684–692 (2012).
Byers, D. E. et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J. Clin. Invest. 123, 3967–3982 (2013). This study identifies the role of an APEC population that releases IL-33 to drive IL-13-dependent airway disease in a post-viral mouse model and in patients with very severe COPD.
Alexander-Brett, J. & Holtzman, M. J. in Mucosal Immunology (eds Cheroutre, H., Kelsall, B., Lambrecht, B. & Russell, M.) (Academic Press, 2014).
Trompette, A. & Karp, C. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457, 585–588 (2009). This study was the first to demonstrate that allergens might use molecular mimicry of TLR ligands to trigger an allergic reaction.
Millien, V. O. et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science 341, 792–796 (2013).
Gitlin, L. et al. Melanoma differentiation-associated gene 5 (MDA5) is involved in the innate immune response to Paramyxoviridae infection in vivo. PLoS Pathog. 6, e10000734 (2010).
Barton, G. M. & Kagan, J. C. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nature Rev. Immunol. 9, 535–542 (2009).
Ioannidis, I., Ye, F., McNally, B., Willette, M. & Flano, E. Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. J. Virol. 87, 3261–3270 (2013).
Groskreutz, D. J. et al. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J. Immunol. 176, 1733–1740 (2006).
Hewson, C. A., Jardine, A., Edwards, M. R., Laza-Stanca, V. & Johnston, S. L. Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J. Virol. 79, 12273–12279 (2005).
Kato, A., Favoreto, S., Avila, P. C. & Schleimer, R. P. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J. Immunol. 179, 1080–1087 (2007).
Guillot, L. et al. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280, 5571–5580 (2005).
Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Heil, F. et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Triantafilou, K. et al. Human rhinovirus recognition in non-immune cells is mediated by Toll-like receptors and MDA-5, which trigger a synergetic pro-inflammatory immune response. Virulence 2, 22–29 (2011).
Wang, T. et al. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nature Med. 10, 1366–1373 (2004).
Le Goffic, R. et al. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2, e53 (2006).
Gowen, B. B. et al. TLR3 deletion limits mortality and disease severity due to Phlebovirus infection. J. Immunol. 177, 6301–6307 (2006).
Hutchens, M. et al. TLR3 increases disease morbidity and mortality from vaccinia infection. J. Immunol. 180, 483–491 (2008).
Rudd, B. D. et al. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J. Immunol. 176, 1937–1942 (2006).
Wang, Q. et al. MDA5 and TLR3 initiate pro-inflammatory signaling pathways leading to rhinovirus-induced airways inflammation and hyperresponsiveness. PLoS Pathog. 7, e1002070 (2011).
Lukacs, N. W. et al. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J. Immunol. 185, 2231–2239 (2010).
Bezemer, G. F. G. et al. Dual role of Toll-like receptors in asthma and chronic obstructive pulmonary disease. Pharmacol. Rev. 64, 337–358 (2012).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature Immunol. 5, 730–737 (2004).
Gitlin, L. et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl Acad. Sci. USA 103, 8459–8464 (2006).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Meylan, E. et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, 1167–1172 (2005).
Seth, R. B., Sun, L., Ea, C. K. & Chen, Z. J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 122, 669–682 (2005).
Xu, L. G. et al. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol. Cell 19, 727–740 (2005).
Ichinohe, T., Lee, H. K., Ogura, Y., Flavell, R. A. & Iwasaki, A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206, 79–87 (2009).
Thomas, P. G. et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30, 566–575 (2009).
Lamkanfi, M. & Dixit, V. M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 28, 137–161 (2012).
Muruve, D. A. et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innnate immune response. Nature 452, 103–107 (2008).
Allen, I. C. et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30, 556–565 (2009).
Allen, I. C. et al. Analysis of NLRP3 in the development of allergic airway disease in mice. J. Immunol. 188, 2884–2893 (2012).
Schoggins, J. W. et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472, 481–485 (2011).
Iversen, M. B. & Paludan, S. R. Mechanisms of type III interferon expression. J. Interferon Cytokine Res. 30, 573–578 (2010).
Osterlund, P. I., Pietila, T. E., Veckman, V., Kotenko, S. V. & Julkunen, I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-λ) genes. J. Immunol. 179, 3434–3442 (2007).
Sommereyns, C., Paul, S., Staeheli, P. & Michiels, T. IFN-lambda (IFN-λ) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 4, e1000017 (2008).
Ioannidis, I. et al. Plasticity and virus specificity of the airway epithelial cell immune response during respiratory virus infection. J. Virol. 86, 5422–5436 (2012).
Okabayashi, T. et al. Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus Res. 160, 360–366 (2011).
Shornick, L. P. et al. Airway epithelial versus immune cell Stat1 function for innate defense against respiratory viral infection. J. Immunol. 180, 3319–3328 (2008).
Wark, P. A. et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 201, 937–947 (2005).
Contoli, M. et al. Role of deficient type III interferon-λ production in asthma exacerbations. Nature Med. 12, 1023–1026 (2006).
Edwards, M. R. et al. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 6, 797–806 (2013).
Djukanovic, R. et al. The effect of inhaled interferon-β on worsening of asthma symptoms caused by viral infections: a randomized trial. Am. J. Respir. Crit. Care Med. 190, 145–154 (2014).
Cakebread, J. A. et al. Exogenous IFN-β has antiviral and anti-inflammatory properties in primary bronchial epithelial cells from asthmatic subjects exposed to rhinovirus. J. Allergy Clin. Immunol. 127, 1148–1154 (2011).
Harada, M. et al. Functional polymorphism in the suppressor of cytokine signaling 1 gene associated with adult asthma. Am. J. Respir. Cell. Mol. Biol. 36, 491–496 (2007).
Torgerson, D. G. et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nature Genet. 43, 887–893 (2011).
Zheng, S. et al. Impaired innate host defense causes susceptibility to respiratory virus infections in cystic fibrosis. Immunity 18, 619–630 (2003).
Schneider, D. et al. Increased cytokine resonse of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 182, 332–340 (2010).
Mallia, P. et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am. J. Respir. Crit. Care Med. 183, 734–742 (2011).
Bochkov, Y. A. et al. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 3, 69–80 (2010).
Lopez-Souza, N. et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J. Allergy Clin. Immunol. 123, 1384–1390 (2009).
DeMore, J. P. et al. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16. J. Allergy Clin. Immunol. 124, 245–252 (2009).
Message, S. D. et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc. Natl Acad. Sci. USA 105, 13562–13567 (2008).
Sampath, D., Castro, M., Look, D. C. & Holtzman, M. J. Constitutive activation of an epithelial signal transducer and activator of transcription (Stat1) pathway in asthma. J. Clin. Invest. 103, 1353–1361 (1999).
Bullens, D. M. et al. Type III IFN-λ mRNA expression in sputum of adult and school-aged asthmatics. Clin. Exp. Allergy 38, 1459–1467 (2008).
Jakiela, B. et al. Th2-type cytokine induced mucous metaplasia decreases susceptibility of human bronchial epithelium to rhinovirus infection. Am. J. Respir. Cell. Mol. Biol. 51, 229–241 (2014).
Patel, D. A. et al. Interferon response and respiratory virus control are preserved in bronchial epithelial cells in asthma. J. Allergy Clin. Immunol. http://dx.doi.org/10.1016/j.jaci.2014.07.013 (2014).
Zhang, Y. et al. Modification of the Stat1 SH2 domain broadly improves interferon efficacy in proportion to p300/CREB-binding protein coactivator recruitment. J. Biol. Chem. 280, 34306–34315 (2005).
Patel, D. A., Patel, A. C., Nolan, W. C., Zhang, Y. & Holtzman, M. J. High throughput screening for small molecule enhancers of the interferon signaling pathway to drive next-generation antiviral drug discovery. PLoS ONE 7, e36594 (2012).
Patel, D. A. et al. High-throughput screening normalized to biological response: application to antiviral drug discovery. J. Biomol. Screen. 19, 119–130 (2014).
Ibricevic, A. et al. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J. Virol. 80, 7469–7480 (2006).
Lachowicz-Scroggins, M. E., Boushey, H. A., Finkbeiner, W. E. & Widdicombe, J. H. Interleukin-13 induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection. Am. J. Respir. Cell. Mol. Biol. 43, 652–661 (2010).
Gu, B. et al. Volatile sensing functions for pulmonary neuroendocrine cells. Am. J. Respir. Cell. Mol. Biol. 50, 637–646 (2014).
Walter, M. J., Morton, J. D., Kajiwara, N., Agapov, E. & Holtzman, M. J. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J. Clin. Invest. 110, 165–175 (2002).
Kim, E. Y. et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic inflammatory lung disease. Nature Med. 14, 633–640 (2008). This study identifies the iNKT cell–macrophage axis that drives chronic lung disease and is characterized by IL-13 production, alternative M2 macrophage activation, airway hyperreactivity and excessive mucus production.
Rawlins, E. L. et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4, 525–534 (2009).
Evans, C. M. et al. Mucin is produced by Clara cells in the proximal airway of antigen-challenged mice. Am. J. Respir. Cell. Mol. Biol. 31, 382–394 (2004).
Rock, J. R. et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl Acad. Sci. USA 106, 12771–12775 (2009).
Patel, A. C. et al. Genetic segregation of airway disease traits despite redundancy of chloride channel calcium-activated family members. Physiol. Genom. 25, 502–513 (2006).
Alevy, Y. et al. IL-13-induced airway mucus production is attenuated by MAPK13 inhibition. J. Clin. Invest. 122, 4555–4568 (2012). This study identifies the IL-13–CLCA1–MAPK13 signalling pathway to excessive mucus production in AECs and provides structure-based drug design for MAPK13 inhibitors that attenuate inflammatory mucus production.
Lefrancais, E. et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc. Natl Acad. Sci. USA 109, 1673–1678 (2012).
Moffat, M. F. et al. A large-scale, consortium-based genomewide association study of asthma. N. Eng. J. Med. 363, 1211–1221 (2010).
Kouzaki, H., Iijima, K., Kobayashi, T., O'Grady, S. M. & Kita, H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J. Immunol. 186, 4375–4387 (2011).
Willart, M. A. et al. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J. Exp. Med. 209, 1505–1517 (2012).
Lommatzsch, M. et al. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 181, 928–934 (2010).
Gauvreau, G. M. et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N. Engl. J. Med. 370, 2102–2110 (2014).
Teisanu, R. M. et al. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am. J. Respir. Cell. Mol. Biol. 44, 794–803 (2011).
Chapman, H. A. et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Invest. 121, 2855–2862 (2011).
McQualter, J. L., Yuen, K., Williams, B. & Bertoncello, I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc. Natl Acad. Sci. USA 107, 1414–1419 (2010).
Kajstura, J. et al. Evidence for human lung stem cells. N. Engl. J. Med. 364, 1795–1806 (2011).
Kumar, P. A. et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147, 525–538 (2011). This study demonstrates that a lung progenitor cell population expands in the setting of influenza virus infection and might participate in alveolar repair.
Papadopoulos, N. G. et al. Viruses and bacteria in acute asthma exacerbations–a GA2LEN-DARE systematic review. Allergy 66, 458–468 (2011).
Kuyper, L. M. et al. Characterization of airway plugging in fatal asthma. Am. J. Med. 115, 6–11 (2003).
Hogg, J. C. et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Eng. J. Med. 350, 2645–2653 (2004).
Yurtsever, Z. et al. Self-cleavage of human CLCA1 by a novel internal metalloprotease domain controls calcium-activated chloride channel activation. J. Biol. Chem. 287, 42138–42149 (2012).
Lisbonne, M. et al. Cutting edge: invariant Vα14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 171, 1637–1641 (2003).
Akbari, O. et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyper-reactivity. Nature Med. 9, 582–588 (2003).
Sen, Y. et al. Vα24-invariant NKT cells from patients with allergic asthma express CCR9 at high frequency and induce Th2 bias of CD3+ T cells upon CD226 engagement. J. Immunol. 175, 4914–4926 (2005).
Akbari, O. et al. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. New. Engl. J. Med. 354, 1117–1129 (2006).
Das, J. et al. Natural killer T cells and CD8+ T cells are dispensable for T cell-dependent allergic airway inflammation. Nature Med. 12, 1345–1346 (2006).
Vijayanand, P. et al. Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. New. Engl. J. Med. 356, 1410–1422 (2007).
Reynolds, C. et al. Natural killer T cells in bronchial biopsies from human allergen challenge model of allergic asthma. J. Allergy Clin. Immunol. 124, 860–862 (2009).
Matangkasombut, P. et al. Natural killer T cells in the lungs of patients with asthma. J. Allergy Clin. Immunol. 123, 1181–1185 (2009).
Brennan, P. J., Brigl, M. & Brenner, M. B. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nature Rev. Immunol. 13, 101–117 (2013).
Albacker, L. A. et al. Invariant natural killer T cells recognize a fungal glycophingolipid that can induce airway hyperreactivity. Nature Med. 19, 1297–1304 (2013).
Parsons, M. W. et al. Dectin-2 regulates the effector phase of house dust mite-elicited pulmonary inflammation independently from its role in sensitization. J. Immunol. 192, 1361–1371 (2014).
Gordon, S. & Martinez, F. O. Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 (2010).
Byers, D. E. & Holtzman, M. J. Alternatively activated macrophages as cause or effect in asthma. Am. J. Respir. Cell. Mol. Biol. 43, 1–4 (2010).
Agapov, E. et al. Macrophage chitinase 1 stratifies chronic obstructive lung disease. Am. J. Respir. Cell. Mol. Biol. 41, 379–384 (2009).
Kaku, Y. et al. Overexpression of CD163, CD204, and CD206 on alveolar macrophages in the lungs of patients with severe chronic obstructive pulmonary disease. PLoS ONE 9, e87400 (2014).
Chaudhuri, R. et al. Sputum matrix metallo-proteinase-12 in patients with chronic obstructive pulmonary disease and asthma: relationship to disease severity. J. Allergy Clin. Immunol. 129, 655–663 (2012).
Spits, H. et al. Innate lymphoid cells — a proposal for uniform nomenclature. Nature Rev. Immunol. 13, 145–149 (2013). This consensus statement provides an approach to categorize ILC1s, ILC2s and ILC3s on the basis of their patterns of receptor expression, cytokine production and pathways for development from a common progenitor cell.
Constantinides, M. G., McDonald, B. D., Verhoef, P. A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Saenz, S. A. et al. IL-25 elicits a multi-potent progenitor cell population that promotes Th2 cytokine responses. Nature 464, 1362–1366 (2010).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+ Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Price, A. E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl Acad. Sci. USA 107, 11489–11494 (2010).
Fallon, P. G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth explusion. J. Exp. Med. 203, 1105–1116 (2006).
Byers, D. E. et al. A distinct population of non-B, non-T (NBNT) cells express high levels of IL-13 during acute and chronic airway disease after viral infection. Am. J. Respir. Crit. Care Med. 179, A4293 (2009).
Barlow, J. L. et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J. Allergy Clin. Immunol. 132, 933–941 (2013).
Monticelli, L. A. et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature Immunol. 12, 1045–1054 (2011).
Barlow, J. L. et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 129, 191–198 (2012).
Bartemes, K. R. et al. IL-33–responsive lineage-CD25+ CD44hi lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J. Immunol. 188, 1503–1513 (2012).
Chang, Y. et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nature Immunol. 12, 631–638 (2011).
Mjosberg, J. M. et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature Immunol. 12, 1055–1162 (2011). This study represents one of the initial efforts to examine ILC2s across several mucosal barriers in humans.
Nussbaum, J. C. et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 (2013).
Barnig, C. et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci. Transl. Med. 5, 174ra26 (2013).
Gregoire, C. et al. The trafficking of natural killer cells. Immunol. Rev. 220, 169–182 (2007).
Jayaraman, A. et al. IL-15 complexes induce NK- and T-cell responses independent of type I IFN signaling during rhinovirus infection. Mucosal Immunol. 7, 1151–1164 (2014).
Fahradi, N. et al. Natural killer cell NKG2D and granzyme B are critical for allergic pulmonary inflammation. J. Allergy Clin. Immunol. 133, 827–835 (2013).
Kim, H. Y. et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nature Med. 20, 54–61 (2014). This study suggests that IL-17-producing ILC3s may contribute to the pathogenesis of obesity-related asthma.
Molofsky, A. B. et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 210, 535–549 (2013).
Hams, E., Locksley, R. M., McKenzie, A. N. & Fallon, P. G. IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J. Immunol. 191, 5349–5353 (2013).
Spencer, S. P. et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 343, 432–437 (2014).
Neves, J. S. & Weller, P. F. Functional extracellular eosinophil granules: novel implications in eosinophil immunobiology. Curr. Opin. Immunol. 21, 694–699 (2009).
Woodruff, P. G. et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc. Natl Acad. Sci. USA 104, 15858–15863 (2007).
Corren, J. et al. Lebrikizumab treatment in adults with asthma. New. Engl. J. Med. 365, 1088–1098 (2011). This study identifies serum periostin as a useful biomarker for patients who might respond to treatment with the IL-13-specific monoclonal antibody lebrikizumab.
Corren, J. Anti-interleukin-5 antibody therapy in asthma and allergies. Curr. Opin. Allergy Clin. Immunol. 11, 565–570 (2011).
Kitaguchi, Y., Komatsu, Y., Fujimoto, K., Hanaoka, M. & Kubo, K. Sputum eosinophilia can predict responsiveness to inhaled corticosteroid treatment in patients with overlap syndrome of COPD and asthma. Int. J. Chron. Obstruct. Pulmon. Dis. 7, 283–289 (2012).
Bafadhel, M. et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am. J. Respir. Crit. Care Med. 186, 48–55 (2012).
Bouffi, C. et al. IL-33 markedly activates murine eosinophils by an NF-κB-dependent mechanism differentially dependent upon an IL-4–driven autoinflammatory loop. J. Immunol. 191, 4317–4325 (2013).
Balzar, S. et al. Mast cell phenotype, location, and activation in severe asthma. Data from the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 183, 299–309 (2011).
Ballarin, A. et al. Mast cell infiltration discriminates between histopathological phenotypes of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 186, 233–239 (2012).
Kawakami, T. & Galli, S. J. Regulation of mast-cell and basophil function and survival by IgE. Nature Rev. Immunol. 2, 773–786 (2002).
Ho, L. H. et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcɛRI signals. J. Leukoc. Biol. 82, 1481–1490 (2007).
Hoenderdos, K. & Condliffe, A. The neutrophil in chronic obstructive pulmonary disease. Am. J. Respir. Cell. Mol. Biol. 48, 531–539 (2013).
O'Byrne, P. M. et al. Neutrophil depletion inhibits airway hyperresponsiveness induced by ozone exposure. Am. Rev. Respir. Dis. 130, 214–219 (1984).
Grayson, M. H. et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J. Exp. Med. 204, 2759–2769 (2007).
Cheung, D. S. et al. Cutting Edge: CD49d+ neutrophils induce FcɛRI expression on lung dendritic cells in a mouse model of postviral asthma. J. Immunol. 185, 4983–4987 (2010).
Busse, W. W. et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N. Engl. J. Med. 364, 1005–1015 (2011).
Manni, M. L., Robinson, K. R. & Alcorn, J. F. A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert Rev. Respir. Med. 8, 25–42 (2014).
Nakae, S., Suto, H., Berry, G. J. & Galli, S. J. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood 109, 3640–3648 (2007).
Hellings, P. W. et al. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am. J. Respir. Cell. Mol. Biol. 28, 42–50 (2003).
Alcorn, J. F., Crowe, C. R. & Kolls, J. K. TH17 cells in asthma and COPD. Annu. Rev. Physiol. 72, 495–516 (2010).
Lajole, S. et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nature Immunol. 11, 928–935 (2010).
Kudo, M. et al. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nature Med. 18, 547–554 (2013).
Busse, W. W. et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am. J. Respir. Crit. Care Med. 188, 1294–1302 (2013).
Moore, W. C. et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J. Allergy Clin. Immunol. 133, 1557–1563 (2014).
Pelaia, G., Vatrella, A. & Maselli, R. The potential of biologics for the treatment of asthma. Nature Rev. Drug Discov. 11, 958–972 (2012).
Holgate, S. T. Trials and tribulations in identifying new biologic treatments for asthma. Trends Immunol. 33, 238–246 (2012).
Busse, W. W., Ring, J., Huss-Marp, J. & Kahn, J. E. A review of treatment with mepolizumab, an anti-IL-5 mAb, in hypereosinophilic syndromes and asthma. J. Allergy Clin. Immunol. 125, 803–813 (2010).
Nair, P. et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N. Engl. J. Med. 360, 985–993 (2009).
Haldar, P. et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 360, 973–984 (2009).
Castro, M. et al. Relizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am. J. Respir. Crit. Care Med. 184, 1125–1132 (2011).
Kolbeck, R. et al. MEDI-563, a humanized anti-IL-5 receptor α mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J. Allergy Clin. Immunol. 125, 1344–1353 (2010).
Laviolette, M. et al. Effect of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J. Allergy Clin. Immunol. 132, 1086–1096 (2013).
Brightling, C. E., She, D., Ranade, K. & Piper, E. Efficacy and safety of tralokinumab, an anti-IL-13 monoclonal antibody, in a Phase 2b study of uncontrolled severe asthma. Am. J. Respir. Crit. Care Med. 189, A6770 (2014).
Piper, E. et al. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur. Respir. J. 41, 330–338 (2013).
Wenzel, S., Wilbraham, D., Fuller, R., Getz, E. B. & Longphre, M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet 370, 1396–1398 (2007).
Slager, R. E. et al. IL-4-receptor polymorphisms predict reduction in asthma exacerbations during response to an anti-IL-4 receptor α antagonist. J. Allergy Clin. Immunol. 130, 516–522 (2012).
Pavord, I. D. et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 380, 651–659 (2012).
Wenzel, S. et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 368, 2455–2466 (2013).
Acknowledgements
The authors sincerely thank the members of the Holtzman laboratory and their collaborators for generating the research perspective that underlies this review. Research on this topic in the laboratory of M.J.H. was supported by grants from the US National Institutes of Health (U19-AI070489, R01-HL121791, U01-AI095776, P01-HL29594 and P50-HL107183).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.J.H. is the principal investigator for research grants to Washington University from Hoffman-LaRoche and Forest Laboratories. D.E.B, J.A.-B. and X.W. declare no competing interests.
Related links
Glossary
- Airway epithelial cells
-
(AECs). The AECs that line the airways include ciliated, mucous, secretory and basal cell types. The AECs also line the alveoli and include type 1 and type 2 epithelial cells.
- Pattern recognition receptors
-
(PRRs). These receptors are a key part of the initial activation step for innate immune cells. They recognize pathogen-associated molecular patterns that are associated with microbial pathogens, as well as damage-associated molecular patterns associated with cell damage.
- Airway progenitor epithelial cell
-
(APEC). These cells are able to proliferate and then differentiate into different airway epithelial cell subsets.
- Innate lymphoid cells
-
(ILCs). A group of cells that are similar in size and shape to lymphocytes but that do not express typical markers of T cells, B cells, natural killer (NK) cells, NKT cells or the granulocyte lineage. A current paradigm divides ILCs into three groups: ILC1s that produce interferon-γ; ILC2s that produce interleukin-5 (IL-5) and IL-13; and ILC3s that produce IL-17 and/or IL-22 and are also known as ILC17s and ILC22s.
- M2-type macrophage activation
-
An immune response that involves the alternative activation of macrophages and monocytes, and is characterized by a gene expression profile that is distinctive of stimulation by interleukin-4 (IL-4) or IL-13. There is still some uncertainty over the best markers for interferon-γ-driven classical activation of M1-type macrophages versus IL-4- and IL-13-driven alternative activation of M2-type macrophages, but the concept remains useful in mouse models of disease and is a starting point for defining macrophage responses in human disease.
- Periostin
-
A ligand for the αVβ3 and αVβ5 integrins that supports epithelial cell migration and adhesion.
- Mutein
-
A mutant form of a protein.
Rights and permissions
About this article
Cite this article
Holtzman, M., Byers, D., Alexander-Brett, J. et al. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat Rev Immunol 14, 686–698 (2014). https://doi.org/10.1038/nri3739
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3739
This article is cited by
-
Direct effects of mast cell proteases, tryptase and chymase, on bronchial epithelial integrity proteins and anti-viral responses
BMC Immunology (2021)
-
Neutralization of IL-33 modifies the type 2 and type 3 inflammatory signature of viral induced asthma exacerbation
Respiratory Research (2021)
-
Oleoylethanolamide induces eosinophilic airway inflammation in bronchial asthma
Experimental & Molecular Medicine (2021)
-
Elevated inflammatory responses and targeted therapeutic intervention in a preclinical mouse model of ataxia-telangiectasia lung disease
Scientific Reports (2021)
-
Cell-specific expression of lung disease risk-related genes in the human small airway epithelium
Respiratory Research (2020)