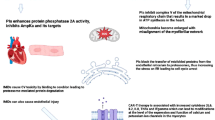
Key Points
-
Advances in treatment have increased the number of childhood cancer survivors with late-occurring, treatment-related cardiovascular complications
-
Treatment-induced cardiovascular events associated with chemotherapy and radiotherapy include cardiomyopathy, conduction defects, myocardial infarction, hypertension, stroke, pulmonary oedema, dyspnoea, and exercise intolerance
-
The likelihood that subclinical damage will progress to clinically important events emphasizes the importance of reducing or eliminating this early damage
-
Protective strategies that have been partially tested include administering cardioprotective agents, using less-toxic anthracycline derivatives and correcting therapy-related endocrinopathies that affect cardiovascular disease development
-
Traditional cardiovascular risk factors, in addition to factors directly related to cancer treatments, can further increase the risk of cardiovascular complications in survivors
-
Cancer treatment protocols should be refined to minimize cardiovascular and other adverse effects while maintaining acceptable cancer treatment response rates
Abstract
Treatment advances and higher participation rates in clinical trials have rapidly increased the number of survivors of childhood cancer. However, chemotherapy and radiation treatments are cardiotoxic and can cause cardiomyopathy, conduction defects, myocardial infarction, hypertension, stroke, pulmonary oedema, dyspnoea and exercise intolerance later in life. These cardiotoxic effects are often progressive and irreversible, emphasizing a need for effective prevention and treatment to reduce or avoid cardiotoxicity. Medical interventions, such as angiotensin-converting enzyme inhibitors, β-blockers, and growth hormone therapy, might be used to treat cardiotoxicity in childhood cancer survivors. Preventative strategies should include the use of dexrazoxane, which provides cardioprotection without reducing the oncological efficacy of doxorubicin chemotherapy; less-toxic anthracycline derivatives and the use of antioxidant nutritional supplements might also be beneficial. Continuous-infusion doxorubicin provides no benefit over bolus infusion in children. Identifying patient-related (for example, obesity and hypertension) and drug-related (for example, cumulative dose) risk factors for cardiotoxicity could help tailor treatments to individual patients. However, all survivors of childhood cancer are at increased risk of cardiotoxicity, suggesting that survivor screening recommendations for assessment of global risk of premature cardiovascular disease should apply to all survivors. Optimal, evidence-based monitoring strategies and multiagent preventative treatments still need to be identified.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
American Cancer Society. Cancer facts & figures 2012. American Cancer Society [online], (2012).
Howlader, N. et al. SEER cancer statistics review, 1975–2010. National Cancer Institute [online], (2013).
Mariotto, A. B. et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol. Biomarkers Prev. 18, 1033–1040 (2009).
Oeffinger, K. C. et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 355, 1572–1582 (2006).
Lipshultz, S. E. et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N. Engl. J. Med. 324, 808–815 (1991).
Mulrooney, D. A. et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 339, b4606 (2009).
Tukenova, M. et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J. Clin. Oncol. 28, 1308–1315 (2010).
Mertens, A. C. et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J. Natl Cancer Inst. 100, 1368–1379 (2008).
Reulen, R. C. et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA 304, 172–179 (2010).
Moller, T. R. et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: a population-based study in the Nordic countries. J. Clin. Oncol. 19, 3173–3181 (2001).
Kalay, N. et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J. Am. Coll. Cardiol. 48, 2258–2262 (2006).
Legha, S. S. et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann. Intern. Med. 96, 133–139 (1982).
Lipshultz, S. E. et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N. Engl. J. Med. 351, 145–153 (2004).
Lipshultz, S. E. et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 11, 950–961 (2010).
Walker, D. M. et al. Dexrazoxane use in pediatric patients with acute lymphoblastic or myeloid leukemia from 1999 and 2009: analysis of a national cohort of patients in the Pediatric Health Information Systems database. Pediatr. Blood Cancer 60, 616–620 (2013).
Lipshultz, S. E. & Adams, M. J. Cardiotoxicity after childhood cancer: beginning with the end in mind. J. Clin. Oncol. 28, 1276–1281 (2010).
Moghrabi, A. et al. Results of the Dana–Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood 109, 896–904 (2007).
Kremer, L. C., van Dalen, E. C., Offringa, M., Ottenkamp, J. & Voûte, P. A. Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J. Clin. Oncol. 19, 191–196 (2001).
Lipshultz, S. E. et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J. Clin. Oncol. 23, 2629–2636 (2005).
Bagnes, C., Panchuk, P. N. & Recondo, G. Antineoplastic chemotherapy induced QTc prolongation. Curr. Drug Saf. 5, 93–96 (2010).
Kilickap, S., Akgul, E., Aksoy, S., Aytemir, K. & Barista, I. Doxorubicin-induced second degree and complete atrioventricular block. Europace 7, 227–230 (2005).
Nakamae, H. et al. QT dispersion correlates with systolic rather than diastolic parameters in patients receiving anthracycline treatment. Intern. Med. 43, 379–387 (2004).
Zhai, L. et al. Long-term results of pirarubicin versus doxorubicin in combination chemotherapy for aggressive non-Hodgkin's lymphoma: single center, 15-year experience. Int. J. Hematol. 91, 78–86 (2010).
Krause, D. S. et al. Tyrosine kinases as targets for cancer therapy. N. Engl. J. Med. 353, 172–187 (2005).
Farolfi, A. et al. Trastuzumab-induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart 99, 634–639 (2013).
Pansy, J. et al. Add-on-therapy with bevacizumab in children and adolescents with poor prognosis non-CNS solid tumors. Anticancer Drugs 24, 198–203 (2013).
Force, T., Krause, D. S. & Van Etten, R. A. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibitors. Nat. Rev. Cancer 7, 332–344 (2007).
Ewer, M. S. et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J. Clin. Oncol. 23, 7820–7826 (2005).
Barry, E., Alvarez, J. A., Scully, R. E., Miller, T. L. & Lipshultz, S. E. Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin. Pharmacother. 8, 1039–1058 (2007).
Ebb, D. et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the Children's Oncology Group. J. Clin. Oncol. 30, 2545–2551 (2012).
Adams, M. J., Hardenbergh, P. H., Constine, L. S. & Lipshultz, S. E. Radiation-associated cardiovascular disease. Crit. Rev. Oncol. Hematol. 45, 55–75 (2003).
Adams, M. J. et al. Radiation-associated cardiovascular disease: manifestations and management. Semin. Radiat. Oncol. 13, 346–356 (2003).
Albini, A. et al. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J. Natl Cancer Inst. 102, 14–25 (2010).
Bensky, A. S., Kothadia, J. M. & Covitz, W. Cardiac effects of dexamethasone in very low birth weight infants. Pediatrics 97, 818–821 (1996).
Adams, M. J. & Lipshultz, S. E. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatr. Blood Cancer 44, 600–606 (2005).
Simbre, V. C., Duffy, S. A., Dadlani, G. H., Miller, T. L. & Lipshultz, S. E. Cardiotoxicity of cancer chemotherapy: implications for children. Paediatr. Drugs 7, 187–202 (2005).
Giantris, A., Abdurrahman, L., Hinkle, A., Asselin, B. & Lipshultz, S. E. Anthracycline-induced cardiotoxicity in children and young adults. Crit. Rev. Oncol. Hematol. 27, 53–68 (1998).
Von Hoff, D. D. et al. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 91, 710–717 (1979).
Lipshultz, S. E. et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N. Engl. J. Med. 332, 1738–1743 (1995).
Orgel, E. et al. Early cardiac outcomes following contemporary treatment for childhood acute myeloid leukemia: a North American perspective. Pediatr. Blood Cancer 60, 1528–1533 (2013).
Hudson, M. M. et al. Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J. Clin. Oncol. 25, 3635–3643 (2007).
Trachtenberg, B. H. et al. Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatr. Cardiol. 32, 342–353 (2011).
Lipshultz, S. E. et al. Continuous versus bolus infusion of doxorubicin in children with ALL: long-term cardiac outcomes. Pediatrics 130, 1003–1011 (2012).
Zhang, J. et al. Sex-related differences in mast cell activity and doxorubicin toxicity: a study in spontaneously hypertensive rats. Toxicol. Pathol. http://dx.doi.org/10.1177/0192623313482778.
Rodvold, K. A., Rushing, D. A. & Tewksbury, D. A. Doxorubicin clearance in the obese. J. Clin. Oncol. 6, 1321–1327 (1988).
Heidenreich, P. A. et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease. J. Clin. Oncol. 25, 43–49 (2007).
van der Pal, H. et al. High risk of symptomatic cardiac events in childhood cancer survivors. J. Clin. Oncol. 30, 1429–1437 (2012).
Landy, D. C. et al. Cranial irradiation as an additional risk factor for anthracycline cardiotoxicity in childhood cancer survivors: an analysis from the cardiac risk factors in childhood cancer survivors study. Pediatr. Cardiol. 34, 826–834 (2013).
Lipshultz, S. E. et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J. Clin. Oncol. 30, 1050–1057 (2012).
Blanco, J. G. et al. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer 112, 2789–2795 (2008).
Lipshultz, S. E. et al. Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer 119, 3555–3562 (2013).
Visscher, H. et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J. Clin. Oncol. 30, 1422–1428 (2012).
Visscher, H. et al. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr. Blood Cancer 60, 1375–1381 (2013).
Blanco, J. G. et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes—a report from the Children's Oncology Group. J. Clin. Oncol. 30, 1415–1421 (2012).
Miranda, C. J. et al. Hfe deficiency increases susceptibility to cardiotoxicity and exacerbates changes in iron metabolism induced by doxorubicin. Blood 102, 2574–2580 (2003).
Krischer, J. P. et al. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J. Clin. Oncol. 15, 1544–1552 (1997).
Lipshultz, S. E. et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J. Clin. Oncol. 30, 1042–1049 (2012).
Centers for Disease Control and Prevention. How much physical activity do children need? Centers for Disease Control and Prevention [online], (2011).
Ness, K. K. et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer 115, 1984–1994 (2009).
Miller, T. L. et al. Characteristics and determinants of adiposity in pediatric cancer survivors. Cancer Epidemiol. Biomarkers Prev. 19, 2013–2022 (2010).
Landy, D. C. et al. Aggregating traditional cardiovascular disease risk factors to assess the cardiometabolic health of childhood cancer survivors: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Am. Heart J. 163, 295–301 (2012).
Miller, A. M. et al. Exercise capacity in long-term survivors of pediatric cancer: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Pediatr. Blood Cancer 60, 663–668 (2013).
Whitaker, R. C., Wright, J. A., Pepe, M. S., Seidel, K. D. & Dietz, W. H. Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 337, 869–873 (1997).
Meacham, L. R. et al. Cardiovascular risk factors in adult survivors of pediatric cancer—a report from the childhood cancer survivor study. Cancer Epidemiol. Biomarkers Prev. 19, 170–181 (2010).
Messiah, S. E., Arheart, K. L., Lopez-Mitnik, G., Lipshultz, S. E. & Miller, T. L. Ethnic group differences in cardiometabolic disease risk factors independent of body mass index among American youth. Obesity (Silver Spring) 21, 424–428 (2013).
Meacham, L. R. et al. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer 103, 1730–1739 (2005).
Nathan, P. C. et al. The prevalence of overweight and obesity in pediatric survivors of cancer. J. Pediatr. 149, 518–525 (2006).
Razzouk, B. I. et al. Obesity in survivors of childhood acute lymphoblastic leukemia and lymphoma. J. Clin. Oncol. 25, 1183–1189 (2007).
Landy, D. C. et al. Dietary quality, caloric intake, and adiposity of childhood cancer survivors and their siblings: an analysis from the cardiac risk factors in childhood cancer survivors study. Nutr. Cancer 65, 547–555 (2013).
Meacham, L. R. et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch. Intern. Med. 169, 1381–1388 (2009).
Neville, K. A., Cohn, R. J., Steinbeck, K. S., Johnston, K. & Walker, J. L. Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. J. Clin. Endocrinol. Metab. 91, 4401–4407 (2006).
Armenian, S. H. et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood 120, 4505–4512 (2012).
Wasilewski-Masker, K. et al. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics 121, e705–e713 (2008).
de Moor, J. S., Puleo, E., Butterfield, R. M., Li, F. P. & Emmons, K. M. Availability of smoking prevention and cessation services for childhood cancer survivors. Cancer Causes Control 18, 423–430 (2007).
Emmons, K. et al. Predictors of smoking initiation and cessation among childhood cancer survivors: a report from the childhood cancer survivor study. J. Clin. Oncol. 20, 1608–1616 (2002).
Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb. Mortal. Wkly Rep. 58, 1227–1232 (2009).
Clarke, S. A. & Eiser, C. Health behaviours in childhood cancer survivors: a systematic review. Eur. J. Cancer 43, 1373–1384 (2007).
Emmons, K. M. et al. Long-term smoking cessation outcomes among childhood cancer survivors in the Partnership for Health Study. J. Clin. Oncol. 27, 52–60 (2009).
Cheitlin, M. D. et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (ACC/AHA/ASE committee to update the 1997 guidelines for the clinical application of echocardiography). J. Am. Soc. Echocardiogr. 16, 1091–1110 (2003).
Steinherz, L. J. et al. Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Children's Cancer Study Group. Pediatrics 89, 942–949 (1992).
Lipshultz, S. E. et al. Monitoring for anthracycline cardiotoxicity. Pediatrics 93, 433–437 (1994).
Children's Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers. Children's Oncology Group [online], (2008).
Lipshultz, S. E. et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation 96, 2641–2648 (1997).
Cardinale, D. et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J. Am. Coll. Cardiol. 36, 517–522 (2000).
Grewal, J. et al. Usefulness of N–terminal pro–brain natriuretic peptide and brain natriuretic peptide to predict cardiovascular outcomes in patients with heart failure and preserved left ventricular ejection fraction. Am. J. Cardiol. 102, 733–737 (2008).
Lipshultz, S. E. & Colan, S. D. Cardiovascular trials in long-term survivors of childhood cancer. J. Clin. Oncol. 22, 769–773 (2004).
Landy, D. C., Miller, T. L., Wilkinson, J. D. & Lipshultz, S. E. in Cancer and the Heart (eds Ewer, M. S. & Yeh, E. T. H.) 132–156 (Peoples Medical Publishing House-USA, 2013).
Grenier, M. A. et al. Angiotensin-converting enzyme inhibitor therapy for ventricular dysfunction in infants, children and adolescents: a review. Prog. Pediatr. Cardiol. 12, 91–111 (2000).
[No authors listed] Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N. Engl. J. Med. 316, 1429–1435 (1987).
Lipshultz, S. E. et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J. Clin. Oncol. 20, 4517–4522 (2002).
Sieswerda, E. et al. Medical interventions for treating anthracycline-induced symptomatic and asymptomatic cardiotoxicity during and after treatment for childhood cancer. Cochrane Database of Systematic Reviews, Issue 9. Art. No.: CD008011. http://dx.doi.org/10.1002/14651858.CD008011.pub2.
Harmon, W., Rusconi, P., Miller, T. L. & Lipshultz, S. E. in American Academy of Pediatrics Textbook of Pediatric Care, 2nd edn Ch. 351 (eds McInerny, T. K., Adam, H. M., Campbell, D. E., Kamat, D. M. & Kelleher, K. J.) American Academy of Pediatrics (in press).
Noori, A. et al. Beta-blockade in adriamycin-induced cardiomyopathy. J. Card. Fail. 6, 115–119 (2000).
El-Shitany, N. A., Tolba, O. A., El-Shanshory, M. R. & El-Hawary, E. E. Protective effect of carvedilol on adriamycin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. J. Card. Fail. 18, 607–613 (2012).
Gupta, M., Steinherz, P. G., Cheung, N. K. & Steinherz, L. Late cardiotoxicity after bolus versus infusion anthracycline therapy for childhood cancers. Med. Pediatr. Oncol. 40, 343–347 (2003).
Levitt, G. A., Dorup, I., Sorensen, K. & Sullivan, I. Does anthracycline administration by infusion in children affect late cardiotoxicity? Br. J. Haematol. 124, 463–468 (2004).
Lyu, Y. L. et al. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 67, 8839–8846 (2007).
van Dalen, E. C., Caron, H. N., Dickinson, H. O. & Kremer, L. C. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database of Systematic Reviews, Issue 6, Art. No.: CD003917. http://dx.doi.org/10.1002/14651858.CD003917.pub4.
Wouters, K. A., Kremer, L. C., Miller, T. L., Herman, E. H. & Lipshultz, S. E. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br. J. Haematol. 131, 561–578 (2005).
Sallan, S. E. & Lipshultz, S. E. Wise up: do not do it without protection! Pediatr. Blood Cancer 45, 872–873 (2005).
Choi, H. S. et al. Dexrazoxane for preventing anthracycline cardiotoxicity in children with solid tumors. J. Korean Med. Sci. 25, 1336–1342 (2010).
Herman, E. K., Zhang, J., Hiraragi, H. & Lipshultz, S. E. Gender is a factor that can impact the severity of doxorubicin (DXR) toxicity in spontaneously hypertensive rats (SHR) [abstract]. FASEB J. 22 (Suppl.), a719.6 (2008).
Thompson, P. A. et al. Impact of body composition on pharmacokinetics of doxorubicin in children: a Glaser Pediatric Research Network study. Cancer Chemother. Pharmacol. 64, 243–251 (2009).
Lipshultz, S. E., Lipsitz, S. R. & Orav, E. J. Dexrazoxane-associated risk for secondary malignancies in pediatric Hodgkin's disease: a claim without compelling evidence. J. Clin. Oncol. 25, 3179 (2007).
Hellmann, K. Dexrazoxane-associated risk for secondary malignancies in pediatric Hodgkin's disease: a claim without evidence. J. Clin. Oncol. 25, 4689–4690 (2007).
Tebbi, C. K. et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J. Clin. Oncol. 25, 493–500 (2007).
Barry, E. V. et al. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J. Clin. Oncol. 26, 1106–1111 (2008).
Vrooman, L. M. et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL Consortium. Eur. J. Cancer 47, 1373–1379 (2011).
Kishi, T. & Folkers, K. Letter: prevention by coenzyme Q10 (NSC-140865) of the inhibition by adriamycin (NSC-123127) of coenzyme Q10 enzymes. Cancer Treat. Rep. 60, 223–224 (1976).
Folkers, K., Brown, R., Judy, W. V. & Morita, M. Survival of cancer patients on therapy with coenzyme Q10. Biochem. Biophys. Res. Commun. 192, 241–245 (1993).
Cortes, E. P., Gupta, M., Chou, C., Amin, V. C. & Folkers, K. Adriamycin cardiotoxicity: early detection by systolic time interval and possible prevention by coenzyme Q10. Cancer Treat. Rep. 62, 887–891 (1978).
Iarussi, D. et al. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Mol. Aspects Med. 15 (Suppl.), s207–s212 (1994).
Hauser, M., Gibson, B. S. & Wilson, N. Diagnosis of anthracycline-induced late cardiomyopathy by exercise-spiroergometry and stress-echocardiography. Eur. J. Pediatr. 160, 607–610 (2001).
Ginsberg, J. P. & Womer, R. B. Preventing organ-specific chemotherapy toxicity. Eur. J. Cancer 41, 2690–2700 (2005).
Ciaccio, M. et al. Vitamin A preserves the cytotoxic activity of adriamycin while counteracting its peroxidative effects in human leukemic cells in vitro. Biochem. Mol. Biol. Int. 34, 329–335 (1994).
Wang, Y. M. et al. Effect of vitamin E against adriamycin-induced toxicity in rabbits. Cancer Res. 40, 1022–1027 (1980).
Doba, T., Burton, G. W. & Ingold, K. U. Antioxidant and co-antioxidant activity of vitamin C. The effect of vitamin C, either alone or in the presence of vitamin E or a water-soluble vitamin E analogue, upon the peroxidation of aqueous multilamellar phospholipid liposomes. Biochim. Biophys. Acta 835, 298–303 (1985).
Shimpo, K. et al. Ascorbic acid and adriamycin toxicity. Am. J. Clin. Nutr. 54 (Suppl. 6), 1298S–1301S (1991).
Plosker, G. L. & Faulds, D. Epirubicin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cancer chemotherapy. Drugs 45, 788–856 (1993).
Goldin, A., Venditti, J. M. & Geran, R. The effectiveness of the anthracycline analog 4′-epidoxorubicin in the treatment of experimental tumors: a review. Invest. New Drugs 3, 3–21 (1985).
Lahtinen, R. et al. Cardiotoxicity of epirubicin and doxorubicin: a double-blind randomized study. Eur. J. Haematol. 46, 301–305 (1991).
Anderlini, P. et al. Idarubicin cardiotoxicity: a retrospective study in acute myeloid leukemia and myelodysplasia. J. Clin. Oncol. 13, 2827–2834 (1995).
Martoni, A. et al. Comparative phase II study of idarubicin versus doxorubicin in advanced breast cancer. Oncology 47, 427–432 (1990).
Shenkenberg, T. D. & Von Hoff, D. D. Mitoxantrone: a new anticancer drug with significant clinical activity. Ann. Intern. Med. 105, 67–81 (1986).
Pellicori, P., Calicchia, A., Lococo, F., Cimino, G. & Torromeo, C. Subclinical anthracycline cardiotoxicity in patients with acute promyelocytic leukemia in long-term remission after the AIDA protocol. Congest. Heart Fail. 18, 217–221 (2012).
Smith, L. A. et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer 10, 337 (2010).
Steiner, R. Increasing exercise in long-term survivors of pediatric cancer and their siblings: should treatment be a family affair? Pediatr. Blood Cancer 60, 529–530 (2013).
Acknowledgements
This article was supported in part by grants from the NIH (HL072705, HL078522, HL053392, CA127642, CA068484, HD052104, AI50274, HD052102, HL087708, HL079233, HL004537, HL087000, HL007188, HL094100, HL095127, HD80002), the Children's Cardiomyopathy Foundation, the Women's Cancer Association, the Lance Armstrong Foundation, the STOP Children's Cancer Foundation, the Scott Howard Fund and the Michael Garil Fund.
Author information
Authors and Affiliations
Contributions
S. E. Lipshultz and T. L. Miller researched data for the article. S. E. Lipshultz, T. R. Cochran, and V. I. Franco made a substantial contribution to discussion of the content and wrote the article, and all authors reviewed or edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lipshultz, S., Cochran, T., Franco, V. et al. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol 10, 697–710 (2013). https://doi.org/10.1038/nrclinonc.2013.195
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2013.195
This article is cited by
-
Research on GGT-responsive drug carrier with active transport effect
Nano Research (2023)
-
Phytochemical analysis and thrombolytic profiling of Costus afer stem fractions
Future Journal of Pharmaceutical Sciences (2022)
-
Impact of DYRK1A Expression on TNNT2 Splicing and Daunorubicin Toxicity in Human iPSC-Derived Cardiomyocytes
Cardiovascular Toxicology (2022)
-
Next Generation Risk Markers in Preventive Cardio-oncology
Current Atherosclerosis Reports (2022)
-
Effect of chronic stress on tumorigenesis and development
Cellular and Molecular Life Sciences (2022)