Abstract
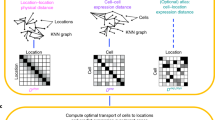
Conventional gene expression studies analyze multiple cells simultaneously or single cells, for which the exact in vivo or in situ position is unknown. Although cellular heterogeneity can be discerned when analyzing single cells, any spatially defined attributes that underpin the heterogeneous nature of the cells cannot be identified. Here, we describe how to use geographical position sequencing (Geo-seq), a method that combines laser capture microdissection (LCM) and single-cell RNA-seq technology. The combination of these two methods enables the elucidation of cellular heterogeneity and spatial variance simultaneously. The Geo-seq protocol allows the profiling of transcriptome information from only a small number cells and retains their native spatial information. This protocol has wide potential applications to address biological and pathological questions of cellular properties such as prospective cell fates, biological function and the gene regulatory network. Geo-seq has been applied to investigate the spatial transcriptome of mouse early embryo, mouse brain, and pathological liver and sperm tissues. The entire protocol from tissue collection and microdissection to sequencing requires ∼5 d, Data analysis takes another 1 or 2 weeks, depending on the amount of data and the speed of the processor.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Change history
13 April 2017
In the version of this article initially published, Shengbao Suo was not indicated as contributing equally to the work with Jun Chen; Jing-Dong J. Han was not indicated as a corresponding author; the expanded form of "Geo-seq" was not given in the abstract; in the heading prior to Step 77, it was not stated that the time indicated excludes the time required for the design of 2D corn plots and for zip-code mapping; "Corn plot visualization and zip-code mapping can be performed through the iTranscriptome portal at http://www.itranscriptome.org." was omitted from the end of Step 77; and some grant numbers were omitted from the Acknowledgments section. These omissions have been corrected in the HTML and PDF versions of the article.
References
Dalerba, P. et al. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nat. Biotechnol. 29, 1120–1127 (2011).
Cann, G.M. et al. mRNA-Seq of single prostate cancer circulating tumor cells reveals recapitulation of gene expression and pathways found in prostate cancer. PLoS One 7, e49144 (2012).
Shalek, A.K. et al. Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature 498, 236–240 (2013).
Buganim, Y. et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell 150, 1209–1222 (2012).
van Wolfswinkel, J.C., Wagner, D.E. & Reddien, P.W. Single-cell analysis reveals functionally distinct classes within the planarian stem cell compartment. Cell Stem Cell 15, 326–339 (2014).
Guo, G. et al. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev. Cell 18, 675–685 (2010).
Yan, L. et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat. Struct. Mol. Biol. 20, 1131–1139 (2013).
Durruthy-Durruthy, R. et al. Reconstruction of the mouse otocyst and early neuroblast lineage at single-cell resolution. Cell 157, 964–978 (2014).
Xue, Z. et al. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 500, 593–597 (2013).
Hebenstreit, D. Methods, challenges and potentials of single cell RNA-seq. Biology 1, 658–667 (2012).
Tang, F. et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Tang, F. et al. RNA-Seq analysis to capture the transcriptome landscape of a single cell. Nat. Protoc. 5, 516–535 (2010).
Hashimshony, T., Wagner, F., Sher, N. & Yanai, I. CEL-Seq: single-cell RNA-Seq by multiplexed linear amplification. Cell Rep. 2, 666–673 (2012).
Islam, S. et al. Highly multiplexed and strand-specific single-cell RNA 5′ end sequencing. Nat. Protoc. 7, 813–828 (2012).
Goetz, J.J. & Trimarchi, J.M. Transcriptome sequencing of single cells with Smart-Seq. Nat. Biotechnol. 30, 763–765 (2012).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
Picelli, S. et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 10, 1096–1098 (2013).
Wen, L. & Tang, F. Single-cell sequencing in stem cell biology. Genome Biol. 17, 71 (2016).
Shapiro, E., Biezuner, T. & Linnarsson, S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet. 14, 618–630 (2013).
Liu, A. Laser capture microdissection in the tissue biorepository. J. Biomol. Tech. 21, 120–125 (2010).
Datta, S. et al. Laser capture microdissection: big data from small samples. Histol. Histopathol. 30, 1255–1269 (2015).
Canas, R.A., Canales, J., Gomez-Maldonado, J., Avila, C. & Canovas, F.M. Transcriptome analysis in maritime pine using laser capture microdissection and 454 pyrosequencing. Tree Physiol. 34, 1278–1288 (2014).
Erickson, H.S. et al. Quantitative RT-PCR gene expression analysis of laser microdissected tissue samples. Nat. Protoc. 4, 902–922 (2009).
Morrison, J.A., Bailey, C.M. & Kulesa, P.M. Gene profiling in the avian embryo using laser capture microdissection and RT-qPCR. Cold Spring Harbor Protoc. 2012, 1249–1262 (2012).
Grover, P.K., Cummins, A.G., Price, T.J., Roberts-Thomson, I.C. & Hardingham, J.E. A simple, cost-effective and flexible method for processing of snap-frozen tissue to prepare large amounts of intact RNA using laser microdissection. Biochimie 94, 2491–2497 (2012).
Zechel, S., Zajac, P., Lonnerberg, P., Ibanez, C.F. & Linnarsson, S. Topographical transcriptome mapping of the mouse medial ganglionic eminence by spatially resolved RNA-seq. Genome Biol. 15, 486 (2014).
Bandyopadhyay, U., Fenton, W.A., Horwich, A.L. & Nagy, M. Production of RNA for transcriptomic analysis from mouse spinal cord motor neuron cell bodies by laser capture microdissection. J. Vis. Exp. 83, e51168 (2014).
Peng, G. et al. Spatial transcriptome for the molecular annotation of lineage fates and cell identity in mid-gastrula mouse embryo. Dev. Cell 36, 681–697 (2016).
Cox, M.L. et al. Assessment of fixatives, fixation, and tissue processing on morphology and RNA integrity. Exp. Mol. Pathol. 80, 183–191 (2006).
Goldsworthy, S.M., Stockton, P.S., Trempus, C.S., Foley, J.F. & Maronpot, R.R. Effects of fixation on RNA extraction and amplification from laser capture microdissected tissue. Mol. Carcinog. 25, 86–91 (1999).
Wang, W.Z., Oeschger, F.M., Lee, S. & Molnar, Z. High quality RNA from multiple brain regions simultaneously acquired by laser capture microdissection. BMC Mol. Biol. 10, 69 (2009).
Parlato, R. et al. A preservation method that allows recovery of intact RNA from tissues dissected by laser capture microdissection. Anal. Biochem. 300, 139–145 (2002).
Wang, H. et al. Histological staining methods preparatory to laser capture microdissection significantly affect the integrity of the cellular RNA. BMC Genomics 7, 97 (2006).
Sonne, S.B. et al. Optimizing staining protocols for laser microdissection of specific cell types from the testis including carcinoma in situ. PLoS One 4, e5536 (2009).
Clement-Ziza, M., Munnich, A., Lyonnet, S., Jaubert, F. & Besmond, C. Stabilization of RNA during laser capture microdissection by performing experiments under argon atmosphere or using ethanol as a solvent in staining solutions. RNA 14, 2698–2704 (2008).
Chomczynski, P. & Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat. Protoc. 1, 581–585 (2006).
Bengtsson, M., Hemberg, M., Rorsman, P. & Stahlberg, A. Quantification of mRNA in single cells and modelling of RT-qPCR induced noise. BMC Mol. Biol. 9, 63 (2008).
Trombetta, J.J. et al. Preparation of single-cell RNA-seq libraries for next generation sequencing. Curr. Protoc. Mol. Biol. 107, 4.22.1–4.22.17 (2014).
Miller, D.F. et al. A new method for stranded whole transcriptome RNA-seq. Methods 63, 126–134 (2013).
Achim, K. et al. High-throughput spatial mapping of single-cell RNA-seq data to tissue of origin. Nat. Biotechnol. 33, 503–509 (2015).
Satija, R., Farrell, J.A., Gennert, D., Schier, A.F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Durruthy-Durruthy, R., Gottlieb, A. & Heller, S. 3D computational reconstruction of tissues with hollow spherical morphologies using single-cell gene expression data. Nat. Protoc. 10, 459–474 (2015).
Junker, J.P. et al. Genome-wide RNA tomography in the zebrafish embryo. Cell 159, 662–675 (2014).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012).
Huber, W., von Heydebreck, A., Sultmann, H., Poustka, A. & Vingron, M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 18, S96–104 (2002).
Zhang, W. et al. Integrating genomic, epigenomic, and transcriptomic features reveals modular signatures underlying poor prognosis in ovarian cancer. Cell Rep. 4, 542–553 (2013).
Acknowledgements
We are grateful to Q. Zhou (Institute of Zoology, Chinese Academy of Sciences) for helpful suggestions, and to Y. Qian of the Jing laboratory for technical support. The sequencing was performed on an Illumina HiSeq2500 system by BerryGenomics (Beijing, China). This work was supported in part by the 'Strategic Priority Research Program' of the Chinese Academy of Sciences (XDA01010201 to N.J., XDB19020301 and XDA01010303 to J.-D.J.H.), the National Key Basic Research and Development Program of China (2014CB964804 and 2015CB964500 to N.J., and 2015CB964803 and 2016YFE0108700 to J.-D.J.H.) and the National Natural Science Foundation of China (31430058, 31571513, 31630043, 91519314 to N.J., and 91329302, 31210103916, 91519330 to J.-D.J.H.).
Author information
Authors and Affiliations
Contributions
N.J. supervised the project. N.J. and G.P. designed the experiment. J.C. and G.P. performed the laser microdissection and RNA-seq. J.C. and S.S. wrote the manuscript. G.P., P.P.L.T., J.-D.J.H. and N.J. edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Methods
Supplementary Methods (PDF 421 kb)
Rights and permissions
About this article
Cite this article
Chen, J., Suo, S., Tam, P. et al. Spatial transcriptomic analysis of cryosectioned tissue samples with Geo-seq. Nat Protoc 12, 566–580 (2017). https://doi.org/10.1038/nprot.2017.003
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.003
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.