Abstract
Eravacycline is an investigational, synthetic fluorocycline antibacterial agent that is structurally similar to tigecycline with two modifications to the D-ring of its tetracycline core: a fluorine atom replaces the dimethylamine moiety at C-7 and a pyrrolidinoacetamido group replaces the 2-tertiary-butyl glycylamido at C-9. Like other tetracyclines, eravacycline inhibits bacterial protein synthesis through binding to the 30S ribosomal subunit. Eravacycline demonstrates broad-spectrum antimicrobial activity against Gram-positive, Gram-negative, and anaerobic bacteria with the exception of Pseudomonas aeruginosa. Eravacycline is two- to fourfold more potent than tigecycline versus Gram-positive cocci and two- to eightfold more potent than tigecycline versus Gram-negative bacilli. Intravenous eravacycline demonstrates linear pharmacokinetics that have been described by a four-compartment model. Oral bioavailability of eravacycline is estimated at 28 % (range 26–32 %) and a single oral dose of 200 mg achieves a maximum plasma concentration (C max) and area under the plasma concentration-time curve from 0 to infinity (AUC0–∞) of 0.23 ± 0.04 mg/L and 3.34 ± 1.11 mg·h/L, respectively. A population pharmacokinetic study of intravenous (IV) eravacycline demonstrated a mean steady-state volume of distribution (V ss) of 320 L or 4.2 L/kg, a mean terminal elimination half-life (t ½) of 48 h, and a mean total clearance (CL) of 13.5 L/h. In a neutropenic murine thigh infection model, the pharmacodynamic parameter that demonstrated the best correlation with antibacterial response was the ratio of area under the plasma concentration-time curve over 24 h to the minimum inhibitory concentration (AUC0–24h/MIC). Several animal model studies including mouse systemic infection, thigh infection, lung infection, and pyelonephritis models have been published and demonstrated the in vivo efficacy of eravacycline. A phase II clinical trial evaluating the efficacy and safety of eravacycline in the treatment of community-acquired complicated intra-abdominal infection (cIAI) has been published as well, and phase III clinical trials in cIAI and complicated urinary tract infection (cUTI) have been completed. The eravacycline phase III program, known as IGNITE (Investigating Gram-Negative Infections Treated with Eravacycline), investigated its safety and efficacy in cIAI (IGNITE 1) and cUTI (IGNITE 2). Eravacycline met the primary endpoint in IGNITE 1, while data analysis for IGNITE 2 is currently ongoing. Common adverse events reported in phase I–III studies included gastrointestinal effects such as nausea and vomiting. Eravacycline is a promising intravenous and oral fluorocycline that may offer an alternative treatment option for patients with serious infections, particularly those caused by multidrug-resistant Gram-negative pathogens.
Similar content being viewed by others
Eravacycline’s potent in vitro activity against a broad-spectrum of Gram-positive and Gram-negative organisms along with intravenous and oral dosing make this new fluorocycline an alternative treatment option for patients with serious infections (particularly those caused by multidrug-resistant pathogens) in both hospital and community settings. |
While eravacycline possesses many qualities of the ideal antimicrobial for treating complicated intra-abdominal infections and complicated urinary tract infections, more clinical efficacy and safety data are required to fully determine its role in treatment of infectious diseases. |
Pharmacoeconomic considerations will also help to further elucidate eravacycline’s place in the clinician’s arsenal of antimicrobials against Gram-positive and Gram-negative infections. |
1 Introduction
The first tetracycline, chlortetracycline, was originally isolated from Streptomyces aureofaciens and introduced into human clinical use in the 1940s [1, 2]. In the 1950s, tetracycline was produced from chlortetracycline by catalytic dehalogenation. The tetracycline class was advanced by the development and market approval of other semisynthetic (second-generation) tetracyclines, doxycycline in late 1960s and minocycline in the early 1970s. Tetracyclines are broad-spectrum antibacterial agents that have been useful in the treatment of a variety of infections ranging from community-acquired respiratory tract infections to less severe conditions such as acne [1, 2]. Unfortunately, their widespread use in human and veterinary medicine as well as in agriculture has led to the development of substantial bacterial resistance and subsequently to decreased utility for indications such as intestinal, respiratory, and urinary tract infections [3, 4]. Efflux pumps and ribosomal protection proteins are the primary mechanisms responsible for resistance to tetracyclines and are present in both Gram-positive and Gram-negative bacteria [1, 5]. Tigecycline, the first marketed intravenous glycylcycline, was developed using semisynthetic methods and received US Food and Drug Administration (FDA) approval in 2005. It represented a major step forward for the tetracycline class as it retained its broad-spectrum activity against both Gram-positive and Gram-negative bacteria including isolates expressing tetracycline efflux pump and ribosomal protection resistance mechanisms [1, 2, 5].
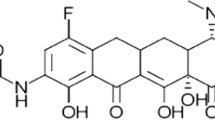
While semisynthetic processes have been important in the development and production of several tetracyclines and glycylcyclines, these methods have an inherent limitation in the diversity of functional groups that can be introduced at C-7 and C-9 of the tetracycline core D-ring (Fig. 1) [6, 7]. This limitation was resolved by the development of a total synthesis route, that allows for a greater array of chemical modifications to be made to the tetracycline core, including substitutions on the D-ring at carbons C-7, C-8, and C-9 [6]. Tetraphase Pharmaceuticals, Inc. (Watertown, MA, USA) has employed this total synthesis process to generate many new tetracycline candidates including the new fluorocycline, eravacycline, previously known as TP-434 [8].
In vitro studies have demonstrated that eravacycline has broad-spectrum activity against both Gram-positive and Gram-negative aerobic and anaerobic pathogens including important antimicrobial resistant pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and extended-spectrum β-lactamase (ESBL)-producing and carbapenemase-producing Enterobacteriaceae and multidrug-resistant Acinetobacter baumannii [9–11]. Similar to tigecycline, eravacycline retains its activity in the presence of the most common tetracycline resistance mechanisms, efflux pumps and ribosomal protection proteins [9, 10].
Both intravenous and oral formulations have been developed for eravacycline [9]. Eravacycline has completed one phase II clinical trial for the treatment of community-acquired complicated intra-abdominal infections (cIAIs) and has completed phase III clinical trials for the treatment of cIAIs and complicated urinary tract infections (cUTIs) [12].
This article reviews existing published data on eravacycline, including relevant chemistry, mechanism of action, mechanisms of resistance, microbiology, pharmacokinetics, pharmacodynamics, efficacy, and safety data from animal and clinical trials. A comprehensive literature search was conducted using MEDLINE, SCOPUS, and databases of scientific meetings from 2005 to October 2015 for all materials containing the terms “eravacycline” or “TP-434.” These results were supplemented by bibliographies obtained from Tetraphase Pharmaceuticals, Inc.
2 Chemistry
The basic chemical structure of tetracyclines is formed by four cycles or rings (A, B, C, and D) and is known as the tetracycline core. Carbons C-1, C-3, C-10, C-11, C-12, and C-12a are substituted with oxygen atoms. C-4 is most commonly a dimethylamine, and C-5, C-6, and C-7 may be replaced with diverse substituents. Eravacycline (7-fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline) is a new tetracycline analog with a fluorine atom at C-7 and a pyrrolidinoacetamido group at C-9 position in the D-ring, distinguishing it from previous tetracyclines and tigecycline (Fig. 1) [6, 13]. The synthesis of eravacycline would not have been practical using the traditional semisynthetic process [6, 8].
Structure-activity relationships (SARs) of tetracyclines are summarized in Fig. 2. The tetracycline derivative, 6-deoxy-6-demethyltetracycline, is the most basic molecule to retain antibacterial activity and is considered the minimum pharmacophore for this structural class [1]. The naturally occurring α stereochemical configuration at C-4a and C-12a and the keto-enol system (C-11 and C-12) close to the D- and A-ring of the tetracycline core ring system are important for the antibacterial activity of tetracyclines [13]. The hydrophilic southern and eastern faces of tetracyclines (C-1, C-2, C-3, C-4, C-10, C-11, C-11a, C-12, and C-12a) cannot be altered without the loss of antibacterial activity [14]. The enolized tricarbonylmethane system at C-1 and C-3 must remain intact for potent activity [14]. The alkylation or replacement of the C-2 amide with functional groups such as aldehydes or nitriles reduces activity [14]. In vitro activity improves when a dimethylamino group at C-4 is in the α-position, and decreases when this group is removed [14]. Furthermore, the addition of alkylamines higher than primary or N-methyl secondary amines, can lead to reduced activity [14]. The cis A/B ring fusion and the β-hydroxyl group at C-12a are required for activity; substitution of the β-hydroxyl group with esters generally leads to inactivity [14]. An enolizable β-diketone at C-11 and C-12 is important as alkylation of C-11 results in inactive products [14]. The formation of a double bond between C-5a and C-11a and the aromatization of the C-ring lead to decreased activity [14].
The C-5, C-5a, C-6, C-7, C-8, and C-9 of the tetracycline core comprise the hydrophobic northern and western faces of tetracycline [14]. This area can undergo modification with retention or even improvement in antibacterial activity [14]. Substitution at C-7 with strong electron withdrawing groups (e.g., chlorine or nitro groups) or electron donating groups (e.g., dimethylamino group) can enhance activity [14]. Tetracycline derivatives lacking a hydroxyl group at C-6 result in greater lipid solubility as demonstrated by the absorption of oral doxycycline and minocycline [14]. The diketone system (C-11 and C-12), enol (C-1 and C-3), and carboxamide (C-2) of tetracyclines act as chelation sites for cations such as calcium, magnesium, and iron, however, is also required for binding to ribosomal RNA [1, 13].
Generally, for fluorocyclines, it has been observed that the more polar or basic substituents attached to the C-9 position (R1 in Fig. 2) result in better antibacterial activity, especially against Gram-negative bacteria [7]. Xiao et al. [6] studied the antibacterial activity of different analogs of 7-fluoro-9-aminoacetamido-6-demethyl-6-deoxytetracyclines against a panel of Gram-positive (S. aureus, Enterococcus faecalis, Streptococcus pneumoniae) and Gram-negative (Escherichia coli, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella pneumoniae) bacteria, including isolates with known tetracycline-resistant genes (S. aureus [tet(M) and tet(K)], E. faecalis [tet(M)], S. pneumoniae [tet(M)], E. coli [tet(A)], and K. pneumoniae [tet(A)]). In general, it was observed that small secondary or tertiary amines at C-9 had a lower minimum inhibitory concentration (MIC) against a majority of the isolates compared to substituents such as aromatic amines and alkylamines with weak basicity. Compared to the tertiary alkylamine analogs, dimethyl, azetidine, and piperidine, the pyrrolidine analog (eravacycline) was eight to 16 times and four to eight times more potent against K. pneumoniae (tet(A)) and E. coli (tet(A)), respectively. Additionally, eravacycline was four- to 64-fold more potent than azetidine and piperidine analogs against all the bacterial isolates tested, with the exception of S. pneumoniae (with and without tet(M)) where equivalent antibacterial activity was observed. Overall, for pneumococci, the addition of polar substituents, fluorine atoms, or bicyclic pyrrolidine analogs showed no improvement or less activity compared to the unsubstituted pyrrolidine analog.
3 Mechanism of Action
Tetracyclines inhibit the elongation phase of protein synthesis by binding to the 30S ribosomal subunit of bacteria (specifically 16S rRNA) and blocking the attachment of aminoacyl tRNA to the acceptor (A) site in the mRNA-ribosome complex [14, 15]. This action prevents amino acid residues from incorporating into the peptide chain, thus inhibiting protein synthesis [1, 15]. This mechanism of action is generally bacteriostatic as the interaction between tetracyclines and ribosomes is reversible [1, 15]. However, eravacycline shows species- and strain-specific cidality. Tetracyclines generally enter Gram-negative bacterial cells through outer membrane porins OmpF and OmpC [1]. It is speculated that tetracyclines pass through these porin channels as magnesium-tetracycline coordination complexes with subsequent dissociation of free tetracycline prior to diffusion through the lipid bilayer of the cytoplasmic membrane [1]. Alternatively, tetracyclines may enter bacterial cells via passive diffusion or active transport, the latter requiring both ATP and Mg2+ for active uptake [14].
Using a [3H]-tetracycline competition assay, Grossman et al. [10] determined the ability of eravacycline, tigecycline, tetracycline, and erythromycin to compete with [3H]-tetracycline in binding to purified 70S ribosomes. In the presence of purified 70S ribosomes and [3H]-tetracycline, increasing concentrations of each unlabeled agent were used to determine its IC50 (the concentration of agent inhibiting 50 % of ribosomes). The IC50s of eravacycline tigecycline and tetracycline were 0.22 ± 0.07, 0.22 ± 0.08, and 3.00 ± 1.15 μM, respectively, and are consistent with eravacycline and tigecycline outcompeting tetracycline for its binding site on the ribosome. As expected, the control agent erythromycin (that binds to the 50S ribosomal subunit) did not compete with [3H]-tetracycline for binding to purified 70S ribosomes.
In another study, investigators used an E. coli-coupled in vitro transcription/translation system that quantitated inhibition via a firefly luciferase readout (luminescence) to assess the inhibition of translation by eravacycline and tetracycline [6]. The IC50 (50 % inhibition of translation compared to untreated controls) was more than seven times lower for eravacycline (0.62 μM) than for tetracycline. The activity of eravacycline, tigecycline, and tetracycline against TetM-protected ribosomes was also tested using the same E. coli-coupled in vitro transcription/translation assay [10]. The average IC50s compared to the untreated controls were 0.29 ± 0.09, 0.08 ± 0.01, and 1.26 ± 0.48 mg/L for eravacycline, tigecycline, and tetracycline, respectively. The average IC50s for eravacycline and tigecycline for TetM-protected ribosomes were equivalent to IC50s for non-TetM-protected ribosomes (0.27 ± 0.16 and 0.09 ± 0.04 mg/L, respectively) whereas the IC50 for tetracycline was fivefold higher for TetM-protected ribosomes. Thus it is clear that eravacycline inhibits translation in both wild-type ribosomes as well as TetM-protected ribosomes.
4 Mechanisms of Resistance
The four mechanisms known to confer tetracycline-specific resistance are efflux pumps, ribosomal protection proteins (RPPs), drug degradation, and rRNA mutations [5]. Of these mechanisms, efflux pumps and RPPs are by far the most prevalent [5]. Some species of Gram-negative bacteria also demonstrate innate resistance to tetracyclines due to specific lipopolysaccharide components in their outer membranes [4, 5]. The tetracycline resistance genes are summarized in Table 1 [1, 4, 5, 16–21].
Tetracycline-specific efflux pumps can be found in the cell membranes of both Gram-positive and Gram-negative bacteria [5, 13]; these are divided into seven groups based primarily on sequence homology [5]. The largest group of efflux pumps are the Group 1, drug-H+ antiporters (e.g., TetA), comprised of 12 transmembrane helices, that transport tetracycline against a concentration gradient through a proton exchange mechanism [5]. Group 1, drug-H+ antiporters are the most common tetracycline resistance mechanism found in Gram-negative bacteria [5]. Although most tetracycline-specific efflux pumps bestow resistance to tetracycline, inconsistent resistance to minocycline, and no resistance to tigecycline or eravacycline [13], certain efflux pumps, such as TetB, confer resistance to tetracycline and minocycline [13]. The expression of genes coding for efflux pumps are frequently regulated by a Tet repressor (TetR) protein that blocks constitutive transcription by binding to upstream operator regions of tet genes [4, 5]. In the presence of tetracycline, TetR preferentially binds to tetracycline, dissociating it from the operator region, thus allowing synthesis of the efflux protein to be initiated [4, 5].
Sutcliffe et al. [11] evaluated the activity of eravacycline against a strain of S. aureus (SA984) demonstrating up-regulated expression of MepA, a multidrug-resistant efflux pump that confers resistance to tigecycline. The MIC of eravacycline increased from 0.004 mg/L (MepA-negative parent isolate, SA983) to 0.016 mg/L in S. aureus expressing MepA compared with an increase in the tigecycline MIC from 0.016 to 1 mg/L (the FDA tigecycline MIC breakpoint for S. aureus is ≤0.5 mg/L). Thus, it does not appear that eravacycline resistance in the clinic will be mediated by mepA.
RPPs sterically weaken interactions between tetracycline and its ribosomal binding site, promoting dissociation of tetracycline and the ribosome, so that amino-acyl tRNAs may bind to the A site, enabling protein synthesis to proceed [4]. RPPs share a high degree of homology with translation elongation factors, EF-Tu, and EF-G GTPases [4]. TetO and TetM are the most prevalent RPPs identified in clinical isolates of Gram-positive and Gram-negative bacteria [4].
Tetracycline inactivation occurs via tet(X) and tet37 gene products that encode for FAD-dependent monooxygenases [5]. These enzymes use NADPH and oxygen to modify tetracyclines through hydroxylation of C-11a (on the tetracycline core), resulting in an unstable compound that undergoes non-enzymatic decomposition [4, 5]. Additionally, the hydroxylated C-11a has been reported to disturb the binding of tetracycline to magnesium that will reduce its affinity for the ribosome [5].
Mutations in the 16S rRNA have also been reported to confer resistance to tetracyclines [5]. The first reported mutation was a point mutation at position 1058 (G1058C E. coli numbering system) in helix 34 of the 16S rRNA in Propionibacterium acnes [4, 5]. An increase in the MIC of tetracycline, doxycycline, and minocycline was reported in P. acnes with three homozygous copies of rRNA with the G1058C mutation [5]. Tetracycline has demonstrated a lower affinity for ribosomes with the G1058C mutation compared to wild-type ribosomes [5]. The G1058 of wild-type ribosomes forms a base-pair interaction with U1199 in helix 34 of 16S rRNA [5]. It is speculated that the disruption caused by the G1058C mutation decreases the affinity of tetracycline to form a coordination complex with Mg2+ due to conformational perturbation of the G1197 and G1198 sites that interact with it [5].
Grossman et al. [10] studied the in vitro activity of eravacycline and comparators against unrelated isolates of Propionibacterium acnes containing the ermX gene (confers erythromycin resistance), a 23S rRNA A2058G mutation (confers macrolide, lincosamide, and streptogramin B resistance) or the previously mentioned 16S rRNA G1058C mutation. In P. acnes isolates with the ermX gene and in ATCC 6919 control strain, the MICs for eravacycline were 0.016 and 0.063 mg/L, respectively. In two non-isogenic P. acnes isolates harboring a 16S rRNA G1058C mutation, the MIC of eravacycline in both isolates was elevated to 1 mg/L. The MIC for tigecycline was 0.5 mg/L for P. acnes ATCC 6919 and the strain carrying the ermX gene, and 2 mg/L in both P. acnes isolates with the 16S rRNA G1058C mutation.
Abdallah et al. [22] identified a significant (P = 0.002) correlation between the MIC of eravacycline and the expression of adeB efflux genes in 38 isolates of Acinetobacter baumannii (MIC range 0.06–4 mg/L) using multiple regression analysis. The disruption of the adeB gene in a hyper-expressing isolate of A. baumannii (40 times greater than control) reduced the eravacycline MIC from 2 to 0.25 mg/L. In isolates with normal expression of adeB (1.4 times greater than control), the MIC of eravacycline remained unchanged (0.25 mg/L) when adeB was disrupted.
Grossman et al. [10] determined the MICs of eravacycline, tigecycline, doxycycline, and minocycline against isogenic strains of E. coli with induced expression of tet(M), tetK, tetB, tetA, or tetX gene mutations. The results were compared against the parental E. coli expressing only lacZ (negative control) to determine antimicrobial activity against the listed resistance mechanisms. The activity of eravacycline remained unchanged against E. coli expressing tet(M) (0.06 mg/L), consistent with the results from the E. coli-coupled in vitro transcription/translation assay previously mentioned in Sect. 3. The MIC of tigecycline increased from 0.06 mg/L (negative control) to 0.13 mg/L against E. coli expressing tet(M). The MICs for eravacycline tested against E. coli expressing tet(K) and tet(B) remained relatively unchanged at 0.03 mg/L and 0.06 mg/L, respectively. As with eravacycline, the MIC for tigecycline against E. coli expressing tet(K) and tet(B) remained unchanged at 0.06 mg/L. For E. coli overexpressing tet(X) (a gene mutation affecting drug degradation), the MIC of eravacycline and tigecycline increased 64- and 32-fold, respectively, compared to the negative control. For E. coli expressing tet(A), the MICs for eravacycline and tigecycline increased by fourfold and 16-fold, respectively, compared to the negative control. The results reported by Grossman et al. [10] suggest that eravacycline is not affected, or only minimally affected, by common efflux pumps (tetA, tetB, tetK) and RPP (tetM) tetracycline resistance genes. The MICs of doxycycline and minocycline tested against the same collection of E. coli isolates increased by 2- to 32-fold, and 2- to 128-fold, respectively, compared to the negative control.
5 Microbiology
Currently available data describing the in vitro activity of eravacycline against Gram-positive, Gram-negative, and anaerobic bacteria are summarized in Tables 2, 3, and 4 [11, 12, 22–36]. The MIC data from various studies were pooled with the MIC range consisting of the lowest and highest recorded MIC values. However, due to the limited availability of eravacycline data for some bacterial species (primarily anaerobes), the results from a single study are presented. Data for comparators were derived from pooled results from studies assessing the activity of eravacycline as well as from previous studies not evaluating eravacycline activity [11, 12, 22–36].
Table 2 depicts the activity of eravacycline against Gram-positive aerobic bacteria, including isolates with common resistance phenotypes. Eravacycline demonstrated MIC50 and MIC90 values ≤0.25 mg/L for all staphylococci, streptococci, and enterococci regardless of concurrent resistance phenotypes (MIC50 and MIC90 values differed by a maximum of twofold) (Table 2). In general, eravacycline was two- to fourfold more active than tigecycline against common clinically important species of Gram-positive aerobic bacteria (Table 2).
Table 3 describes the MIC50 and MIC90 values of eravacycline and comparators against Gram-negative aerobic bacteria with various resistance phenotypes or genotypes. In general, the presence of an ESBL or a non-susceptible phenotype for a third-generation cephalosporin or carbapenem in E. cloacae, K. pneumoniae, and Proteus mirabilis increased the MIC90 by no more than twofold compared to susceptible isolates. For most species of Gram-negative aerobic bacteria, eravacycline was two- to fourfold more potent than tigecycline (Table 3).
Dubois et al. [37] evaluated the in vitro activity of eravacycline against Legionella pneumophila serotypes 1–6 by testing on buffered charcoal yeast extract (BCYE) agar. The mean MIC50 and MIC90 values from all six serotypes were 1 and 2 mg/L, respectively, with a range of 0.015 to 2 mg/L. The same authors also performed a pilot study to determine if BCYE agar supplemented with iron (ferric pyrophosphate) affected the activity of eravacycline [37]. For E.coli ATCC 25922 incubated for 24 h, there was a 16-fold increase in the eravacycline MIC when testing on BCYE agar as compared to cation-adjusted Mueller Hinton broth (CAMHB); for BCYE without ferric pyrophosphate, the MIC on BCYE increased by fourfold compared to CAMHB. The authors of the study concluded that the antibacterial activity of eravacycline was suppressed by the use of BCYE agar. The activity of eravacycline was also determined against Francisella tularensis, Yersinia pestis, and Bacillus anthracis with reported MIC50/MIC90 values of 0.12/0.5, 0.06/0.12, and ≤0.016/0.016 mg/L, respectively [38, 39]. In this same study, the MIC50/MIC90 values of eravacycline against Burkholderia mallei and Burkholderia pseudomallei were 0.06/0.25 and 1/2 mg/L, respectively [38].
Table 4 shows the activity of eravacycline against different Gram-positive and Gram-negative anaerobic bacteria. Due to the limited data on the activity of eravacycline against anaerobic bacteria, the data in Table 4 have not been pooled, with the exception of Bacteroides fragilis. In general, eravacycline demonstrated MIC50 and MIC90 values two- to eightfold more potent than tigecycline for most anaerobic species with the exception of Clostridium perfringens, Bacteroides ovatis, and Fusobacterium spp. where tigecycline was two- to fourfold more active than eravacycline. Currently, no data are available on the activity of eravacycline against atypical pathogens such as Chlamydophila pneumoniae, Mycoplasma pneumoniae, and Rickettsia spp.
Grossman et al. [40] evaluated the in vitro activity of eravacycline in pre-established biofilms formed by a uropathogenic E. coli isolate harboring the tetracycline efflux pump gene tet(B) and the β-lactamase gene bla TEM. After formation of the biofilm, planktonic cells were aseptically aspirated and planktonic cell, biofilm, and macrodilution MICs were determined for eravacycline and comparator antimicrobial agents. A biofilm MIC was determined to be the lowest antimicrobial concentration that demonstrated both a lack of staining with crystal violet and a 90 % reduction in the biofilm. Eravacycline displayed planktonic and biofilm MIC values of 0.25 and 0.5 mg/L, respectively, both similar to results of macrodilution testing (MIC, 0.25 mg/L).
6 Pharmacokinetics
The results of phase 1 clinical trials describing oral eravacycline plasma concentrations are summarized in Table 5 [41, 42]. The pharmacokinetic parameters of intravenous tigecycline and eravacycline are compared in Table 6 [41–45].
Leighton et al. [41] evaluated the pharmacokinetics of eravacycline administered as an oral solution in a single dose of 50, 100, 200, or 300 mg to 24 healthy subjects (six subjects per dose). The bioavailability was determined using the area under the plasma concentration-time curve from 0 to infinity (AUC0–∞) value for each oral dose compared to a mean AUC0–∞ value of 8.98 mg·h/L for a single IV dose of 1.5 mg/kg. Based on data normalized to average body weight (78 kg), Leighton et al. [41] estimated an oral bioavailability of 28 % (range, 26–32 %) with the oral solution of eravacycline. The authors also reported a median time to maximum plasma concentration (t max) value of 2–2.5 h for the four oral doses studied [41]. The maximum plasma concentration (C max) and AUC0–∞ values with the 200-mg dose were 0.23 ± 0.04 mg/L and 3.34 ± 1.11 mg·h/L, respectively. Data for the other doses are provided in Table 5.
The multiple-dose pharmacokinetics of eravacycline were described by Horn et al. [42] in a study of 18 healthy subjects who received oral doses of 100 mg every 12 h, 300 mg every 24 h, or 400 mg every 24 h for 7 days. Due to gastrointestinal intolerance (nausea and vomiting), the pharmacokinetic parameters on day seven were not determined in the 400-mg dose group. On day seven, the mean C max values were 0.16 ± 0.04 and 0.34 ± 0.08 mg/L with 100 mg every 12 h and 300 mg every 24 h, respectively. The terminal AUC values calculated from the last dose on day seven to infinity were 3.08 ± 0.7 and 9.51 ± 2.45 mg·h/L with 100 mg every 12 h and 300 mg every 24 h, respectively.
Yue et al. [44] conducted a population pharmacokinetic analysis of IV eravacycline using data from single-dose (n = 42) and multiple-dose (n = 24) studies in healthy subjects. Single doses of 0.1–3 mg/kg were infused over 30 min whereas multiple doses were administered over 10 days as 0.5 mg/kg infused over 30 min once daily, 1.5 mg/kg infused over 30 or 60 min once daily, or 1 mg/kg infused over 60 min twice daily. Intravenous eravacycline was best characterized by a four-compartment model with linear pharmacokinetics. For the single-dose study, the population mean pharmacokinetic parameters included a steady-state volume of distribution (V ss) of 262 L or 3.3 L/kg, a mean terminal elimination half-life (t ½) of 26 h, and a mean total clearance (CL) of 13.9 L/h. For the multiple-dose study, the V ss was 320 L or 4.2 L/kg, the mean terminal elimination t ½ was 48 h and the mean total CL was 13.5 L/h. The authors proposed that differences in the terminal elimination t ½ were due to longer sampling times in the multiple-dose study. In comparison, the phase I trials of oral eravacycline reported mean t ½ values of 16–27 h and 18–36 h in the single-dose and multiple-dose studies, respectively [41, 42].
Eravacycline studies in healthy volunteers report mean renal clearances of 3.0–3.5 L/h with approximately 16 % of the drug excreted unchanged in the urine [41, 44]. Sutcliffe et al. [46] also measured urine concentrations of eravacycline in healthy subjects. For subjects who received multiple IV doses of 1.5 mg/kg once daily (n = 6), eravacycline concentrations in urine collected from 0–8 h were 9.9 ± 2.9 mg/L (30-min infusion) and 6.9 ± 1.2 mg/L (60-min infusion) on day one and 8.6 ± 8.3 mg/L (30-min infusion) and 13.3 ± 3.4 mg/L (60-min infusion) on day 10. Sutcliffe et al. [46] also found that in subjects who received a single oral dose of 200 mg (n = 6), eravacycline concentrations were 5.5 ± 1.8 mg/L in urine collected from 0–8 h and 5.9 ± 0.96 mg/L in urine collected from 8–24 h [46]. Eravacycline doses of 1.5 mg/kg (IV) and 200 mg (oral) demonstrated comparable plasma exposures and urine concentrations in the lead-in portion of the phase III cUTI (IGNITE 2) trial, and the dosing regimens were carried forward in the pivotal portion of the trial [47].
Singh et al. [43] measured the protein binding of eravacycline in pooled human plasma using microdialysis methods. Atypical non-linear, concentration-dependent protein binding was observed (as has been reported in tigecycline), and at concentrations of 0.1 and 10 mg/L, the free fraction of eravacycline was 20.7 ± 3.7 and 10.5 ± 2.0 %, respectively. Connors et al. [48] further studied the pulmonary disposition of eravacycline in a phase I study of 20 healthy volunteers who received IV doses of 1 mg/kg infused over 60 min every 12 h for seven doses. The mean plasma total AUC0–12h value was 4.56 ± 0.94 mg·h/L with an estimated free AUC0–12h value of 0.77 ± 0.14 mg·h/L based upon the protein binding data generated by Singh et al. [43]. The AUC0–12h value in epithelial lining fluid (ELF) was determined from bronchoalveolar lavage (BAL) fluid samples collected from five patients using measurements made at sampling times of 2, 4, 6, and 12 h. The AUC0–12h value in ELF was 4.93 mg·h/L with an estimated penetration ratio (ELF AUC0–12h value to plasma free AUC0–12h value) of 6.44. However, the authors also noted that eravacycline concentrations in BAL fluid were below the lower limit of quantification (5 ng/mL) in two out of five subjects at 6 h and three out of five subjects at 12 h.
7 Pharmacodynamics
Pharmacodynamics (PD) describe the relationship between antimicrobial exposure and microbiological and/or clinical response, thus understanding PD is useful in selecting optimal antimicrobial dosing in the treatment of infectious diseases [49]. For the tetracyclines, the AUC over 24 h value divided by the MIC value of the pathogen (AUC24h/MIC) has demonstrated the strongest association with outcome [49–51]. For tigecycline, AUC24h/MIC values of 17.9 and 6.96 were significant thresholds in achieving positive clinical outcomes in phase II and III studies for patients with complicated skin and soft tissue infections and complicated intra-abdominal infections, respectively [50, 51]. The PD analysis of eravacycline in patients is ongoing.
Weiss et al. [52] studied the pharmacodynamics of eravacycline in a neutropenic murine thigh infection model. Mice infected with tetracycline-resistant MRSA were treated with eravacycline value of 0.12 mg/L, at doses of 1–90 mg/kg/day administered every 6, 12, or 24 h. Bacterial counts in thigh tissue were determined at 26 h post-infection. The correlation coefficients for the various pharmacodynamic indices [i.e., total AUC24h/MIC, C max/MIC (ratio of maximum drug concentration to an isolate’s MIC value), and %T >MIC (percentage of time drug concentration remain above an isolate’s MIC value)] versus antibacterial response were 82, 80, and 58 %, respectively. AUC24h/MIC thresholds of 38.4 and 46.9 were reported for bacteriostasis and 1 log10 bacterial kill, respectively. Based upon a population pharmacokinetic model using K. pneumoniae (eravacycline MIC50, 0.5 mg/L) and dosing regimens of 1.5 mg/kg infused over 60 min once daily and 1 mg/kg infused over 30 min twice daily, Yue et al. [44] predicted the regimens would achieve total AUC24h/MIC50 values of 15.1 and 20.1, respectively.
The antibacterial activity of eravacycline has also been described using in vitro time-kill assays for 12 isolates of A. baumannii (MIC range, 0.015–2 mg/L), 12 isolates of E. coli (MIC range, 0.03–0.5 mg/L), and 13 isolates of K. pneumoniae (MIC range, 0.25–1 mg/L) with various resistance genotypes (ESBL and tet genes) and resistance phenotypes (carbapenem-resistant) [33]. In general, eravacycline at two to eight times the MIC was bacteriostatic (defined as ≤1 log10 CFU growth over 24 h). Bactericidal effects at relatively high concentrations ranging from 1 to 16, 0.25 to 2, and 0.5 to 8 mg/L were reported against some isolates of A. baumannii (eight of 12 isolates), E. coli (five of 12 isolates), and K. pneumoniae (four of 13 isolates), respectively.
8 Animal Studies
The in vivo efficacy of eravacycline in the treatment of Gram-positive and Gram-negative infections has been evaluated in various animal models. These studies have been summarized in Table 7 [39, 53–55].
Grossman et al. [53] evaluated the efficacy of eravacycline using a mouse systemic infection model with isolates of S. aureus [methicillin-resistant S. aureus (MRSA); methicillin- susceptible S. aureus (MSSA)], S. pyogenes, and E. coli. Mice (n = 6) were infected by intraperitoneal injection and were administered single intravenous doses of eravacycline, tigecycline, and tetracycline (0.05–10 mg/kg) 1 h post-infection. The protective antimicrobial dose for 50 % of the infected animals (PD50) were determined 48 h post-infection and are summarized in Table 7. Against MSSA, MRSA (tet(M)), and MRSA (tet(K)) the PD50 values [95 % confidence intervals (CIs)] for eravacycline were 0.30 mg/kg (0.29–0.31), 1.0 mg/kg (0.56–1.4), and 0.3 mg/kg (0.13–0.47), respectively. In both MRSA isolates, eravacycline and tigecycline had similar PD50 values with overlapping 95 % CIs. For S. pyogenes ATCC 8668, the PD50 values (95 % CIs) for eravacycline and tigecycline were 1 mg/kg (0.78–1.2) and 2.5 mg/kg (1.7–3.4), respectively, with no overlap in CIs. For S. pyogenes strain ATCC 19615, the PD50 value (95 % CIs) of eravacycline was 0.05 mg/kg whereas tigecycline demonstrated a higher PD50 value (95 % CI) of 0.3 mg/kg (0.04–0.56). For E. coli, both eravacycline and tigecycline produced PD50 values (95 % CIs) of 4.4 mg/kg (−0.01–8.7) and 1.7 mg/kg (0.91–2.6), with overlapping 95 % CIs. An ESBL-producing E. coli harboring tetracycline efflux pumps (tet(B) and tet(D)), demonstrated PD50 values of 1.2 mg/kg (0.84–1.6) and 3.5 mg/kg (2.4–4.7) for eravacycline and tigecycline, respectively. Similar PD50 values for eravacycline (1.3 mg/kg) and tigecycline (3.5 mg/kg) were reported in exploratory studies of eravacycline by Tetraphase Pharmaceuticals, Inc. using the same ESBL-producing isolate of E. coli [6].
In a mouse thigh infection model, neutropenic mice (n = 4) were used to determine the antibacterial activity of eravacycline against isolates of S. aureus and S. pyogenes [53]. At 1.5 h post-infection, mice were administered single IV doses of eravacycline ranging from 0.3 to 30 mg/kg. A linear line plot was used to determine the dose required to produce 1 log10, 2 log10, and 3 log10 CFU reductions relative to 24-h untreated controls. For S. aureus, eravacycline doses of 0.2, 0.2, and 0.4 mg/kg were required to generate 1 log10, 2 log10, and 3 log10 CFU reductions, respectively. In contrast, tigecycline required doses of 1.2, 1.5, and 2 mg/kg, respectively, to produce the same effects. Similar results were observed when MRSA (tet(M)) isolates were tested with eravacycline, displaying 5-, 12.5- and 6-fold greater potencies than tigecycline to produce 1 log10, 2 log10, and 3 log10 CFU reductions, respectively. In contrast, similar doses of eravacycline (3.5 and 9.5 mg/kg) and tigecycline (3.8 and 8 mg/kg) were required to produce 1 log10 and 2 log10 reductions in another isolate of MRSA (tet(K)), respectively. A maximum of a 2.1 log10 CFU reduction was achieved using eravacycline at 10 mg/kg. For a 1 log10 CFU reduction in S. aureus (tet(K)), similar doses of eravacycline (2.3 mg/kg) and tigecycline (2.1 mg/kg) were required. Eravacycline at doses of 8.2 and 16.2 mg/kg provided 2 log10 and 3 log10 CFU reductions, respectively; this was 1.5-fold less potent than tigecycline. With S. pyogenes, 3 and 9 mg/kg of eravacycline reduced the CFU by 1 and 2 log10, respectively, with a maximum 2.3 log10 CFU reduction at 10 mg/kg. In the same model, 6 and 15.8 mg/kg of tigecycline produced 1 and 2 log10 CFU reductions, respectively, with a maximum 2.3 log10 CFU reduction at 20 mg/kg.
Xiao et al. [6] also evaluated the in vivo efficacy of eravacycline versus MRSA (tet(M)) in a murine neutropenic thigh model. At 1.5 h post-infection, IV eravacycline at 0.6 or 3 mg/kg was administered and produced 1 log10 and 3 log10 reductions in bacterial burden relative to 24-h post-treatment controls, respectively. In comparison, tigecycline at doses of 3 and 17.3 mg/kg produced 1 log10 and 3 log10 CFU reductions, respectively.
The in vivo efficacy of eravacycline against uropathogenic, tetracycline-resistant E. coli was studied using a mouse pyelonephritis model [53]. Mouse kidneys (n = 4–6) were infected with E. coli EC200 (1.3 × 108 CFU) followed by 2, 5, or 10 mg/kg eravacycline administered at 12 and 24 h post-infection. At 36 h post-infection, eravacycline at 2, 5, and 10 mg/kg demonstrated log10 CFU reductions of 1.3, 3.8, and 4.6, respectively, compared to untreated controls. The log10 CFU reductions were significant for eravacycline dosed at 2 mg/kg (P < 0.01), 5 mg/kg (P < 0.01), and 10 mg/kg (P < 0.05). Murphy et al. [50] presented results from the same study with an ESBL-producing K. pneumoniae strain. They reported log10 CFU reductions of 1.6 and 2.4 at 36 h for eravacycline doses of 10 and 20 mg/kg IV BID (two doses), respectively. This suggests that at an equipotent dose of 10 mg/kg BID, eravacycline efficacy is considerably less (1000× higher kidney CFU counts) for K. pneumoniae.
Grossman et al. [53] also described the efficacy of eravacycline and comparators (linezolid and vancomycin) against S. pneumoniae and MRSA (tet(M)) in a mouse lung infection model. Infected neutropenic mice (n = 5–6) were treated with eravacycline or comparators two and 12 h post-infection. The lung bacterial density was determined 26 h post-infection. For S. pneumoniae, IV eravacycline doses of 3, 6, and 12 mg/kg produced significant (P < 0.01) log10 CFU reductions of 2.6, 3.1, and 3.9, respectively. In the MRSA lung infection model, 10 mg/kg of IV eravacycline produced a CFU reduction of 2.4 log10 similar to 30 mg/kg of oral linezolid whereas 50 mg/kg of IV vancomycin produced a 1.4 log10 CFU reduction.
Sutcliffe et al. [39] described the efficacy of eravacycline against F. tularensis-infected cynomolgus monkeys. Cynomolgus monkeys received aerosolized F. tularensis and treatment commenced when elevated temperature readings were measured in nine consecutive 15-min intervals; animals were dosed with eravacycline within 6 h of the last elevated temperature reading. The study examined treatment with eravacycline using humanized doses of 8 and 12 mg/kg/day versus a saline control for 21 days followed by monitoring until 14 days post-treatment. In both groups treated with eravacycline, there was a 100 % survival rate (n = 8/8 and n = 7/7) whereas the survival rate in the saline-treated controls was 25 % (n = 2/8). In the deceased subset of the control group, the lung, liver, spleen, and all but one mediastinal lymph nodes were positive for F. tularensis. Additionally, five of the six non-survivors of the control group yielded positive F. tularensis terminal blood cultures. All animals treated with eravacycline resolved their fever within ~2 days of treatment initiation and remained non-bacteremic throughout the study; the blood cultures for the survivors of the control group were also negative during the course of the study. In the eravacycline treatment groups and the two survivors of the control group, samples of lung, liver, mediastinal lymph nodes, and spleen were negative for F. tularensis.
Sutcliffe et al. [54] reported the in vivo efficacy of eravacycline against Bacillus anthracis in rabbits. In the study, rabbits (n = 24) were exposed to 2 × 107 CFU of B. anthracis Ames spores and monitored hourly for fever development. Animals were treated when fever persisted for three consecutive hourly intervals, or were serum-positive for protective antigen. Within 6 h of either event, animals received their first dose of eravacycline (0.8 or 1.6 mg/kg/day) humanized to provide exposures equivalent to 1.5 mg/kg every 24 h (q24 h) or 1.0 mg/kg every 23 h (q12 h), respectively. Of the rabbits, 95.8 % (23 of 24) were confirmed to be bacteremic before treatment initiation. In both eravacycline groups (0.8 and 1.6 mg/kg/day), all of the rabbits survived the 28-day treatment period and the 28-day post-treatment period, whereas all saline control rabbits died within 4–5 days post-infection. In the eravacycline treatment groups, blood samples were negative for B. anthracis at 24, 30, 36, 42, and 48 h into treatment; on day 28 post-infection; and on days 1, 2, 3, 7, 14, and 21 post-treatment.
9 Clinical Trials
At this time, the single phase II clinical trial evaluating the efficacy and safety of eravacycline in the treatment of community-acquired intra-abdominal infections (cIAI) has been published, and the phase III program known as IGNITE (Investigating Gram-negative Infections Treated with Eravacycline), to investigate the safety and efficacy of eravacycline in cIAI (IGNITE 1) and complicate urinary tract infection (cUTI) (IGNITE 2), has been completed. Preliminary results from IGNITE 1 as well as the Lead-In portion of IGNITE 2 have been presented [47, 56].
The safety and efficacy of eravacycline in the treatment of adults with community-acquired cIAI was evaluated in a phase II randomized, double-blind trial (NCT01265784) (Table 8) [12]. The purpose of the study was to provide preliminary safety and efficacy data and thus it was not statistically powered to demonstrate non-inferiority of eravacycline to the comparator ertapenem. This study included 18- to 75-year-old male and female patients with a body mass index of ≤30 kg/m2 who were not expected to require antimicrobial therapy for more than 14 days. A diagnosis of cIAI necessitating urgent surgical or percutaneous intervention was required for enrollment. Acceptable diagnoses included appendiceal perforation, peri-appendiceal abscess, diverticulitis abscess, acute gastric and duodenal perforation (operated after 24 h of perforation), traumatic perforation of the intestines (operated after 12 h of perforation), and/or abscess or peritonitis caused by perforated viscus, or any other intra-abdominal abscesses with the exclusion of the liver and spleen. Of note, the study enforced an enrollment cap of ≤50 % diagnoses of complicated appendicitis. Exclusion criteria in this study included diagnostic symptoms of complicated appendicitis for less than 24 h before present hospitalization, hospitalization within 6 months prior to screening, inflammatory bowel disease including suspected/known disease or associated visceral abscess, management by open abdominal techniques including staged abdominal repair, Acute Physiology and Chronic Health Evaluation (APACHE) II score of >25, rapidly progressing or life threatening illness, probable death before the end of the study, therapeutic dosages of vasopressors required to maintain a systolic or diastolic blood pressure of ≥90 or ≥70 mmHg, respectively, renal failure, abnormal renal function, and abnormal liver function. Additionally, patients treated with systemic antimicrobials for more than 24 h, patients who received carbapenems or tigecycline for the current infection, or required any other systemic antimicrobials were excluded.
In the study, treatment regimens included 1.0 mg/kg IV eravacycline every 12 h infused over 60 min, 1.5 mg/kg IV eravacycline every 24 h infused over 60 min or 1 g IV ertapenem every 24 h infused over 30 min with patients randomized in a 2:2:1 ratio (Table 8). Antimicrobial therapy was continued for 4–14 days depending on the clinical status of the patient. The primary endpoint of the study was clinical response in the microbiologically evaluable (ME) population at the test of cure evaluation (10–14 days post-treatment) (Table 8). Clinical response was classified as cure, failure, or indeterminate. Cure was defined as resolution or significant improvement in signs and symptoms of the initial infection with no requirement of additional therapies (antibacterial, surgical, or radiological). Failure was defined as an intra-abdominal infection-related death, documented infection persisting or recurring within the abdomen, post-surgical wound infection or use of any effective concomitant antimicrobial(s) during the treatment, regardless of the indication. In the study, eravacycline treatment at 1.5 mg/kg q24 h and 1 mg/kg q12 h, and ertapenem at 1 g q24 h demonstrated cure rates in the ME population of 92.9 % (95 % CI 80.5–98.5), 100 % (95 % CI 91.4–100) and 92.3 % (95 % CI 74.9–99.1), respectively (Table 8). Failures in the eravacycline 1.5 mg/kg treatment group included two patients who required additional antimicrobial agents (for treatment of lobar pneumonia and persistent fever) and one patient who had a fatal thromboembolism. The baseline pathogens identified were Gemella morbillorum in the patient with lobar pneumonia and P. aeruginosa and K. pneumoniae in the patient with persistent fever. The two clinical failures for ertapenem were attributed to a newly developed subphrenic abscess in one patient (with baseline pathogens K. pneumoniae, Acinetobacter spp., and Streptococcus salivarius), and an allergic reaction requiring alternative antimicrobial therapy in another patient.
Secondary outcomes of the study included the microbiological response in the ME and microbiologically modified intention to treat (m-MITT) population with outcomes defined as favorable (eradication or presumed eradication of the pathogen), unfavorable (persistence or presumed persistence of the pathogen), or indeterminate at the end of treatment and TOC (test of cure). Results for the microbiological outcome in the ME population are listed in Table 8. In the assessment of the safety and tolerability, two patients in the eravacycline 1.5 mg/kg treatment group and two patients in the ertapenem 1 g treatment group required discontinuation of their study antimicrobials due to a treatment emergent adverse effect; further details regarding adverse effects are described in Sect. 10.
Recently, preliminary efficacy and safety data of eravacycline in the treatment of adults with cIAI was presented from a phase III randomized, double-blind trial [56]. The purpose of the study was to provide efficacy and safety data of eravacycline to the comparator ertapenem. This study included adults patients (age ≥18 years) with a mean age of ~55 years, males/female ~57/43 %, mostly caucasians (~96 %) with APACHE scores of 0–10 (~83 % of patients). A diagnosis of cIAI necessitating urgent surgical or percutaneous intervention was required for enrollment. The majority of patients were diagnosed with complicated appendicitis, intra-abdominal abscess, peritonitis or complicated cholecystitis. 1298 organisms from 446 patients (~three isolates/patient) were obtained with E. coli (58 %), Bacteroides spp. (38 %), Streptococcus spp. (29 %), and E. faecalis (20 %) as the most common organisms.
In the study, treatment regimens included 1.0 mg/kg IV eravacycline every 12 h and 1 g IV ertapenem administered every 24 h. Antimicrobial therapy was continued for 4–14 days depending on the clinical status of the patient. The primary endpoint of the study was clinical response in the microbiologically evaluable (ME) population at the test of cure evaluation (10–14 days post-treatment). Clinical response was classified as cure, failure, or indeterminate. Cure was defined as resolution or significant improvement in signs and symptoms of the initial infection with no requirement of additional therapies (antibacterial, surgical, or radiological). Failure was defined as an intra-abdominal infection-related death, documented infection persisting or recurring within the abdomen, post-surgical wound infection or use of any effective concomitant antimicrobial(s) during the treatment, regardless of the indication. In the study, eravacycline treatment at 1.0 q12 h and ertapenem at 1 g q24 h demonstrated cure rates in the ME population of 92.9 % (222/239) and 94.5 % (225/238), respectively. Clinical failures occurred in 7.1 and 5.5 % in the eravacycline and ertapenem arms, respectively.
Secondary outcomes of the study included the clinical cure in the ME and microbiologically modified intention to treat (m-MITT) population with outcomes defined as favorable (eradication or presumed eradication of the pathogen), unfavorable (persistence or presumed persistence of the pathogen), or indeterminate at the end of treatment and TOC (test of cure). Eravacycline treatment at 1.0 q12 h and ertapenem at 1 g q24 h demonstrated clinical cure rates in the ME population of 87.0 % (235/270) and 88.8 % (238/268), respectively. Eravacycline treatment at 1.0 q12 h and ertapenem at 1 g q24 h demonstrated clinical cure rates in the m-MITT population of 86.8 % (191/220) and 87.6 % (198/226), respectively. No significant differences between eravacycline or ertapenem in clinical outcome or microbiological outcome were reported in this study [56].
Limited data are also available from the phase III clinical trial IGNITE 2, assessing the efficacy of eravacycline versus levofloxacin in the treatment of cUTI [47]. This trial enrolled 980 patients who were randomized 1:1 to receive eravacycline (1.5 mg/kg IV every 24 h followed by 200 mg orally every 12 h) or levofloxacin (750 mg IV every 24 h followed by 750 mg every 24 h) [47]. Each patient received a minimum of 3 days of IV dosing and then, if clinically indicated were eligible to transition to oral therapy for the remaining doses for a total treatment of 7 days. For the FDA the primary analysis evaluated responder outcome (a combination of clinical cure rate and microbiological response) in the microbiological intent to treat (micro-ITT) population at the post-treatment (PT) visit (defined as 6–8 days after completion of therapy) using a 10 % non-inferiority margin. For the European Medicines Agency (EMA) the primary analysis evaluated the microbiological response in the microbiologically modified ITT (micro-MITT) population and microbiologically evaluable (ME) populations at the PT visit using a 10 % non-inferiority margin. Tetraphase recently concluded that using either the FDA or EMA analysis, the IGNITE2 phase III clinical trial of eravacycline administered as an IV to oral therapy for the treatment of cUTI did not achieve its primary endpoint of statistical non-inferiority compared to IV/PO levofloxacin [47]. More analysis is ongoing to assess the reason(s) for these results.
10 Adverse Effects
The safety and tolerability of eravacycline has been evaluated in phase I–III clinical trials. The adverse events are discussed below and summarized in Table 9 [12, 41, 42, 47, 48, 56].
Preliminary safety and tolerability of eravacycline was evaluated in a phase I oral single dose study in three groups of six healthy volunteers receiving eravacycline, 200 or 300 mg or placebo, respectively [41]. A total of seven of 24 subjects experienced an adverse event such as nausea, vomiting, dizziness, increased alanine aminotransferase, and increased unconjugated bilirubin (both <1.2 of the upper limit of normal). Prolonged activated partial thromboplastin times (<1.5 of the upper limit of normal) were reported in five subjects in the 300-mg single-dose group and in two subjects in the placebo group. No adverse events were reported in the 50- and 100-mg treatment groups. Overall, adverse events were reported to be self-limiting.
The safety and tolerability of eravacycline was also assessed in an oral multiple-ascending dose study phase I clinical trial involving three groups of six healthy subjects receiving doses of 100 mg twice daily, 300 mg once daily, or 400 mg once daily, respectively, for 7 days [42]. Adverse events reported by two or more subjects in any study group included nausea, vomiting, abdominal pain, upper abdominal pain, and headache. In subjects receiving 400 mg of eravacycline, treatment was discontinued on day five due to tolerability issues related to nausea and vomiting (Table 9). A higher incidence of nausea and vomiting was reported in the 400-mg treatment group compared to the other treatment groups. No safety signals were found upon review of vital signs, electrocardiogram, physical examinations, and laboratory values testing for chemistry, hematology, urinalysis, coagulation, liver function, and renal function.
The safety and tolerability of intravenous eravacycline administered 1.0 mg/kg twice daily for seven doses in 20 healthy volunteers were evaluated in a pulmonary disposition study [48]. A total of 78 adverse events were reported from 19 of 20 volunteers, of which 64 adverse events were determined to be related to eravacycline use. The adverse events were reported as mild 70.5 % (55 of 78) or moderate 29.5 % (23 of 78). Reported side effects included nausea, vomiting, headache, and irritation related to infusion. No subjects discontinued drug therapy due to adverse events. In the three phase I studies no serious adverse events (SAEs) were reported [41, 42, 48].
In a phase II trial assessing the safety and efficacy of intravenous eravacycline 1.5 mg/kg daily or 1 mg/kg twice daily versus ertapenem 1 g once daily in the treatment of cIAI, treatment emergent adverse events (TEAEs) were reported in 35.8 % (19 of 53), 28.6 % (16 of 56), and 26.7 % (8 of 30), respectively, among the three treatment groups [12]. The most common TEAEs were nausea (1.9 and 10.7 % for eravacycline 1.5 and 1 mg/kg, respectively and 6.7 % for ertapenem) and vomiting (5.7, 1.8, and 0 %, respectively). Drug treatment was discontinued in two patients in the eravacycline 1.5 mg/kg dosage group and one patient in the ertapenem group. SAEs were reported in three, one, and one patients receiving eravacycline 1.5, eravacycline 1 mg/kg, and ertapenem 1 g, respectively. No SAEs were considered related to the study drug. Three of the six SAEs reported in patients receiving eravacycline 1.5 mg/kg were deaths attributed to duodenal ulcer hemorrhage, atrial fibrillation, and embolism. No safety signals were identified through laboratory tests, physical examinations, vital sign measurements, or electrocardiogram [12].
In a phase III trial assessing the safety and efficacy of intravenous eravacycline 1.0 mg/kg every 12 h versus ertapenem 1 g once daily in the treatment of cIAI, TEAEs were reported in 14.8 % (40/270) and 1.5 % (4/268), respectively, among the two treatment groups [56]. The most common TEAEs for eravacycline and ertapenem were nausea (3.3 and 0.4 %) and vomiting (2.2 and 0 %) respectively. Vascular disorders were reported in 8.1 and 2.6 % of patients receiving eravacycline and ertapenem, respectively. SAEs were reported in 17 and 16 patients receiving eravacycline and ertapenem, respectively. Deaths due to any cause occurred in 1.1 and 2.2 % of patients receiving eravacycline and ertapenem, respectively. Data analysis from IGNITE 2, assessing the safety of eravacycline compared to levofloxacin in the treatment of cUTI is currently ongoing [47].
So far in clinical trials it appears that nausea with or without vomiting was reported with eravacycline more commonly than comparators. At this time the safety data with eravacycline is relatively limited and further studies are required to fully assess the adverse effect profile associated with eravacycline.
11 Drug Interactions
Presently, limited data are available on drug interactions involving eravacycline. Therefore, this section will briefly summarize known drug interactions for the tetracycline class.
Tetracyclines contain chemical functional groups that form insoluble chelates with cations such as calcium, magnesium, and iron [14, 57, 58]. Concurrent use of oral tetracyclines and antacids containing aluminum, calcium and magnesium may impair the absorption of oral tetracyclines leading to decreased serum concentrations [57–59]. If used concomitantly, it is recommended to administer tetracycline 2–4 h before or 2–6 h after an antacid to minimize the interaction [57–59]. Iron salts and tetracyclines may decrease the absorption of both agents possibly through chelation [57–60]. To minimize this interaction, administer iron salts 3 h before and 2 h after tetracycline [58, 59]. Antidiarrheal agents such as bismuth subsalicylate may decrease absorption of tetracyclines; alternative therapy is recommended [58, 59].
Tetracyclines may increase plasma concentrations of anticoagulants such as warfarin causing an enhancement of its anticoagulant effect [59, 60]. Monitoring of anticoagulant activity through international normalized ratio or prothrombin time is recommended with appropriate drug adjustments [57–59].
Concomitant use of tetracyclines with digoxin may increase concentrations of digoxin in less than 10 % of patients due to reduced metabolism by the gastrointestinal flora and may persist several months after discontinuation of tetracycline therapy [57–59]. Patients should be monitored for potential digoxin toxicity (increased concentrations, nausea, arrhythmias) and their dose reduced as necessary [57–59]. Anti-epileptic drugs such as barbiturates, carbamazepine, and phenytoin may decrease serum concentrations of doxycycline possibly through increased hepatic metabolism [57–60]. Whether these agents interact with eravacycline (16–35 % renal excretion) is unknown [44]. In vitro human hepatocyte studies have evaluated the metabolic stability of eravacycline [61]. After incubation for 4 h with human hepatocytes, the percentage of eravacycline remaining was 85.3 % [61].
Concurrent use of tetracyclines may reduce the efficacy of oral contraceptives; however, this remains controversial due to limited evidence [57–59]. Regardless, it is recommended that additional forms of contraception be used during tetracycline therapy [57–59]. Concomitant use of tetracyclines and penicillins may decrease the bactericidal effects of penicillin leading to drug antagonistic [57–60]. This combination should be avoided when possible [50, 53]. Use of retinoids (isotrentoin or acitretin) with tetracyclines may increase risk of pseudotumor cerebri and is generally not recommended [55, 59, 60].
12 Place of Eravacycline Therapy
Eravacycline demonstrates broad-spectrum antimicrobial activity against Gram-positive, Gram-negative, and anaerobic bacteria with the exception of P. aeruginosa. Eravacycline is two- to fourfold more active than tigecycline versus Gram-positive cocci and two- to eightfold more active versus Gram-negative bacilli. Unlike tigecycline, eravacycline is being evaluated in both oral and parenteral formulations. Oral eravacycline may allow for step-down therapy from intravenous eravacycline or other broad-spectrum intravenous therapy and may permit its use beyond the hospital setting. In both phase II and III clinical trials, IV eravacycline demonstrated safety and efficacy in the treatment of cIAIs. Completed phase I, II, and III clinical trials have demonstrated the safety of eravacycline; however, the more data are required to fully assess the adverse event profile and drug interactions of eravacycline. Eravacycline is a promising new oral and intravenous fluorocycline.
References
Zhanel GG, Homenuik K, Nichol K, et al. The glycylcyclines: a comparative review with the tetracyclines. Drugs. 2004;64(1):63–88.
Nelson ML, Levy SB. The history of the tetracyclines. Ann N Y Acad Sci. 2011;1241:17–32.
Bassetti M, Merelli M, Temperoni C, et al. New antibiotics for bad bugs: where are we? Ann Clin Microbiol Antimicrob. 2013;12:22.
Thaker M, Spanogiannopoulos P, Wright GD. The tetracycline resistome. Cell Mol Life Sci. 2010;67(3):419–31.
Nguyen F, Starosta AL, Arenz S, et al. Tetracycline antibiotics and resistance mechanisms. Biol Chem. 2014;395(5):559–75.
Xiao XY, Hunt DK, Zhou J, et al. Fluorocyclines. 1. 7-fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline: a potent, broad spectrum antibacterial agent. J Med Chem. 2012;55(2):597–605.
Clark RB, Hunt DK, He M, et al. Fluorocyclines. 2. Optimization of the C-9 side-chain for antibacterial activity and oral efficacy. J Med Chem. 2012;55(2):606–22.
Ronn M, Zhu Z, Hogan PC, et al. Process R&D of eravacycline: the first fully synthetic fluorocycline in clinical development. Org Process Res Dev. 2013;17(5):838–45.
Bassetti M, Righi E. Eravacycline for the treatment of intra-abdominal infections. Expert Opin Investig Drugs. 2014;23(11):1575–84.
Grossman TH, Starosta AL, Fyfe C, et al. Target- and resistance-based mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob Agents Chemother. 2012;56(5):2559–64.
Sutcliffe JA, O’Brien W, Fyfe C, et al. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother. 2013;57(11):5548–58.
Solomkin JS, Ramesh MK, Cesnauskas G, et al. Phase 2, randomized, double-blind study of the efficacy and safety of two dose regimens of eravacycline versus ertapenem for adult community-acquired complicated intra-abdominal infections. Antimicrob Agents Chemother. 2014;58(4):1847–54.
Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65(2):232–60.
Beale JM. Antibacterial Antibiotics. In: Beale JM, Block JH, editors. Wilson and Gisvold’s textbook of organic medicinal and pharmaceutical chemistry. 12th ed. Baltimore: Lippincott Williams & Wilkins; 2011. p. 258–329.
Brenner GM, Stevens CW. Inhibitors of bacterial protein synthesis. In: Brenner GM, Stevens CW, editors. Pharmacology. 4th ed. Philadelphia: Elsevier Saunders; 2013. p. 408–16.
Roberts MC. Update on acquired tetracycline resistance genes. FEMS Microbiol Lett. 2005;245(2):195–203.
Kazimierczak KA, Rincon MT, Patterson AJ, et al. A new tetracycline efflux gene, tet(40), is located in tandem with tet(O/32/O) in a human gut firmicute bacterium and in metagenomic library clones. Antimicrob Agents Chemother. 2008;52(11):4001–9.
Thompson SA, Maani EV, Lindell AH, et al. Novel tetracycline resistance determinant isolated from an environmental strain of Serratia marcescens. Appl Environ Microbiol. 2007;73(7):2199–206.
Brown MG, Mitchell EH, Balkwill DL. Tet 42, a novel tetracycline resistance determinant isolated from deep terrestrial subsurface bacteria. Antimicrob Agents Chemother. 2008;52(12):4518–21.
You Y, Hilpert M, Ward MJ. Identification of Tet45, a tetracycline efflux pump, from a poultry-litter-exposed soil isolate and persistence of tet(45) in the soil. J Antimicrob Chemother. 2013;68(9):1962–9.
Warburton PJ, Ciric L, Lerner A, et al. TetAB46, a predicted heterodimeric ABC transporter conferring tetracycline resistance in Streptococcus australis isolated from the oral cavity. J Antimicrob Chemother. 2013;68(1):17–22.
Abdallah M, Olafisoye O, Cortes C, et al. Activity of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including multidrug-resistant isolates, from New York City. Antimicrob Agents Chemother. 2015;59(3):1802–5.
Zhanel GG, Adam H, Baxter M, et al. Activity of eravacycline and comparators against 3,174 pathogens isolated from Canadian hospitals: CANWARD; 2014.
Hackel M, Bouchillon S, Biedenbach D, et al. Comparative analysis of eravacycline (TP-434) by Broth microdilution and disk diffusion [abstract no. E-1180 plus poster]. 53rd annual interscience conference on antimicrobial agents and chemotherapy; 10–13 Sep 2013; Denver.
Sutcliffe J, O’Brien W, Achorn C, et al. In vitro activity of fluorocycline TP-434 against panels of recent bacterial clinical isolates [abstract no. F1-2158 plus poster]. 50th annual interscience conference on antimicrobial agents and chemotherapy; 12–15 Sep 2010; Boston.
Hunt D, Xiao X, Clark R, et al. TP-434 is a novel broad-spectrum fluorocycline [abstract no. F1-2157 plus poster]. 50th annual interscience conference on antimicrobial agents and chemotherapy; 12–15 Sep 2010; Boston.
Zhanel GG, Karlowsky JA, Rubinstein E, et al. Tigecycline: a novel glycylcycline antibiotic. Expert Rev Anti Infect Ther. 2006;4(1):9–25.
Zhanel GG, Johanson C, Embil JM, et al. Ertapenem: review of a new carbapenem. Expert Rev Anti Infect Ther. 2005;3(1):23–39.
Gin A, Dilay L, Karlowsky JA, et al. Piperacillin-tazobactam: a beta-lactam/beta-lactamase inhibitor combination. Expert Rev Anti Infect Ther. 2007;5(3):365–83.
Zhanel GG, Adam HJ, Baxter MR, et al. Antimicrobial susceptibility of 22746 pathogens from Canadian hospitals: results of the CANWARD 2007-11 study. J Antimicrob Chemother. 2013;68(Suppl 1):i7–22.
Morrisey I, Sutcliffe J, Hackel M, Hawser S. Assessment of eravacycline against 3467 recent gram-positive bacteria including multidrug resistant isolates collected from 2013–2014 [abstract no. C-563 plus poster]. 55th annual interscience conference on antimicrobial agents and chemotherapy; 17–21 Sep 2015; San Diego.
Fyfe C, Grossman T, O’Brien W, et al. The novel broad-spectrum fluorocycline TP-434 is active against MDR Gram-negative pathogens [abstract no P1149 plus poster]. 21st European Congress of Clinical Microbiology and Infectious Diseases/27th International Congress of Chemotherapy; 7–10 May 2011; Milan.
Grossman TH, O’Brien W, Fyfe C, et al. Eravacycline is potent against third generation cephalosporin- and carbapenem-resistant Enterobacteriaceae, carbapenem-resistant Acinetobacter baumannii, and has isolate-specific bactericidal activity [abstract no. C-1374 plus poster]. 54th annual interscience conference on antimicrobial agents and chemotherapy; 5–9 Sep 2014; Washington.
Kerstein K, Fyfe C, Sutcliffe JA, et al. Eravacycline (TP-434) is active against susceptible and multidrug-resistant Neisseria gonorrhoeae [abstract no. E-1181 plus poster]. 53rd annual interscience conference on antimicrobial agents and chemotherapy; 10–13 Sep 2013; Denver.
Morrisey I, Sutcliffe J, Hackel M, Hawser S. Activity of eravacycline against anaerobic bacteria from europe collected in 2013–2014 [abstract no. 784 plus poster]. ID Week; 7–11 Oct 2015; San Diego.
McDermott LA, Jacobus NV, Snydman DR, et al. In vitro activity of eravacycline against a broad spectrum of recent clinical anaerobic isolates [abstract no. 1417 plus poster]. ID Week; 17–21 Sep 2015; San Diego.
Dubois J, Dubois M, Martel JF, et al. In vitro activity of fluorocyclines against Legionella pneumophilia [abstract no. F1-2159 plus poster]. 50th annual interscience conference on antimicrobial agents and chemotherapy; 12–15 Sep 2010; Boston.
Hershfield J, Howlett A, Schweizer HP, et al. Eravacycline is potent against category A and B pathogens [abstract no. 114G plus poster]. ASM biodefense and emerging diseases; 25–27 Feb 2013; Washington.
Sutcliffe J, Selden J, Gooldy M, et al. Eravacycline is efficacious in a Francisella tularensis-infected cynomolgus monkey model [abstract no. 094G plus poster]. American Society of Microbiology Biodefense and Emerging Diseases; 27–29 Jan 2014; Washington.
Grossman TH, O’Brien W, Kerstein KO, et al. Eravacycline (TP-434) is active in vitro against biofilms formed by uropathogenic Escherichia coli. Antimicrob Agents Chemother. 2015;59(4):2446–9.
Leighton A, Zupanets I, Bezugla N, et al. Broad-spectrum fluorocycline TP-434 has oral bioavailability in humans [abstract no. P 1509 plus poster]. 21st European Congress of Clinical Microbiology and Infectious Diseases/27th International Congress of Chemotherapy; 7–10 May 2011; Milan.
Horn PT, Sutcliffe JA, Walpole SM, et al. Pharmacokinetics, safety and tolerability of a novel fluorocycline, TP-434 following multiple dose administration. [abstract no. 603 plus poster]. 49th Annual Infectious Diseases Society of America; 20–23 Oct 2011; Boston.
Singh RSP, Falcao NMS, Sutcliffe J, et al. Plasma Protein binding of eravacycline in mouse, rat, rabbit, cynomolgus monkey, African green monkey and human using microdialysis [abstract no. A-015 plus poster]. 53rd annual interscience conference on antimicrobial agents and chemotherapy; 10–13 Sep 2013; Denver.
Yue CS, Sutcliffe JA, Colucci P, et al. Population pharmacokinetics modeling of TP-434, a novel fluorocycline, following single and multiple dose administration [abstract no. A1-028 plus poster]. 50th annual interscience conference on antimicrobial agents and chemotherapy; 12–15 Sep; 2010 Boston.
Pfizer. Tygacil: product monograph. 2014; http://www.pfizer.ca/sites/g/files/g10017036/f/201410/TYGACIL_PM_E_168980_17Jan2014.pdf. Accessed 30 July 2015.
Sutcliffe J, Grossman T, Ronn M, et al. TP-434 has potential to treat complicated urinary tract infections (cUTI) [abstract no. F1-1858 plus poster]. 51th annual interscience conference on antimicrobial agents and chemotherapy; 17–20 Sep 2011; Chicago.
Tsai L, March A, Lloyd K, et al. Results of the lead-in study on the safety and efficacy of eravacycline versus levofloxacin in complicated urinary tract infection [abstract no. 0198]. 25th European Congress of Clinical Microbiology and Infectious Diseases; 25–28 Apr; 2015 Copenhagen.
Connors KP, Housman ST, Pope JS, et al. Phase I, open-label, safety and pharmacokinetic study to assess bronchopulmonary disposition of intravenous eravacycline in healthy men and women. Antimicrob Agents Chemother. 2014;58(4):2113–8.
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–12.
Passarell JA, Meagher AK, Liolios K, et al. Exposure-response analyses of tigecycline efficacy in patients with complicated intra-abdominal infections. Antimicrob Agents Chemother. 2008;52(1):204–10.
Meagher AK, Passarell JA, Cirincione BB, et al. Exposure-response analyses of tigecycline efficacy in patients with complicated skin and skin-structure infections. Antimicrob Agents Chemother. 2007;51(6):1939–45.
Weiss WJ, Pulse M, Renick P, et al. Efficacy of fluorocyclines TP-434 in the neutropenic thigh infection model is predicted by AUC/MIC [abstract no. F1-2164 plus poster]. 50th Annual interscience conference on antimicrobial agents and chemotherapy; 12–15 Sep 2010; Boston.
Grossman TH, Murphy TM, Slee AM, et al. Eravacycline (TP-434) is efficacious in animal models of infection. Antimicrob Agents Chemother. 2015;59(5):2567–71.
Sutcliffe J, Selden J, Radcliff A, et al. Eravacycline protects in a Bacillus anthracis-infected New Zealand white rabbit treatment model [abstract no. P0109 plus poster]. 24th European Congress of Clinical Microbiology and Infectious Diseases; 10–13 May 2014; Barcelona.
Murphy T, Slee A, Sutcliffe J. TP-434 is Highly efficacious in animal models of infection [abstract no. F1-2161 plus poster]. 50th annual interscience conference on antimicrobial agents and chemotherapy (ICAAC), 12–15, Sep 2010; Boston.
Solomkin J, Evans SD, Slepavicius A, et al. Results of IGNITE 1: a phase 3 study to evaluate the efficacy and safety of eravacycline versus ertapenem in complicated intra-abdomial infections [abstract no. 4174]. 25th European Congress of Clinical Microbiology and Infectious Diseases; 25–28 Apr; 2015 Copenhagen.
Drug Interaction Facts. The authority on drug interactions. St. Louis: Lippincott Williams & Wilkins; 2012. p. 2011.
Hansten PD, Horn JR. Drug interactions analysis and management. 8th ed. St. Louis: Lippincott Williams & Wilkins; 2013.
Canadian Pharmacists Association. Compendium of pharmaceuticals and specialties. Ottawa: Canadian Pharmacists Association; 2015.
Drug information handbook. 22nd ed. Hudson: Lexi-Comp Inc.; 2013.
Christ D, Sutcliffe J. TP-434 is metabolicallly stable and has low potential for drug–drug interactions [abstract no. F1-2162 plus poster]. 50th Annual interscience conference on antimicrobial agents and chemotherapy; 12–15 Sep 2010; Boston.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Zhanel has received research grants from Tetraphase Pharmaceuticals Inc. Drs. Cheung, Adam, Zelenitsky, Golden, Schweizer, Gorityala, Lagacé-Wiens, Walkty, Gin, Hoban and Karlowsky have no conflicts of interest to declare.
The authors are grateful to Tetraphase Pharmaceuticals for their assistance with literature retrieval and an unrestricted research grant.
Rights and permissions
About this article
Cite this article
Zhanel, G.G., Cheung, D., Adam, H. et al. Review of Eravacycline, a Novel Fluorocycline Antibacterial Agent. Drugs 76, 567–588 (2016). https://doi.org/10.1007/s40265-016-0545-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0545-8