Abstract
Background
Recent immigrants from developing countries (<2 years since immigration) are at very high risk of active TB disease due to reactivation of latent infections acquired in the country of origin. In industrialized low-incidence TB countries targeted testing programs for high risk groups could allow the detection of latently infected persons who would likely benefit from a course of preventive treatment. In this study we evaluated the tuberculin skin test (TST) and interferon-γ enzyme-linked immunosorbent assay (QuantiFERON TB-gold in tube, QFT-IT) strategies for TB infection screening programs in recent immigrants from highly endemic countries.
Patients and methods
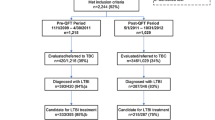
This is a prospective cross-sectional study. Paired tests performed in 1,130 immigrants attending an outpatient ward, between 2005 and 2007 for any health problem were evaluated by intention-to-treat (ITT) and per-protocol (PP) analysis for efficiency and efficacy of screening program.
Results
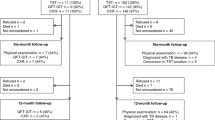
Positive TST and QFT-IT were observed in 36.04 versus 29.82% (ITT) and in 45.27 versus 30.22% (PP) respectively. A higher drop-out rate was observed for TST (20.35 vs. 1.33%) (p < 0.0001). Second level assessment was accepted by half of the TST positive patients. Overall agreement rate between 887 paired tests was fair (k = 0.38). Higher k values were observed for higher TB prevalence rate in the country of origin (k = 0.43), for TST induration diameters >20 mM (k = 0.47), in subjects aged 40–50 years (k = 0.41) and in unvaccinated persons (k = 0.40). In a multiple logistic regression model continent of origin, class of TB prevalence in the country of origin and contacts with TB patients were found to be significantly associated with the probability of TST and QFT-IT positive result. Low education levels were associated only to an increased risk of TST positive results.
Conclusions
The drawback of the TST screening strategy in recent immigrants from highly endemic countries is due to low sensitivity/specificity of the test and to high drop-out rate with an overall significant lowering in strategy efficacy/efficiency. The higher QFT-IT specificity prevents unnecessary overload of the health care system and, although more expensive, might represent a cost-effective alternative to TST in targeted screening programs directed to high risk populations.

Similar content being viewed by others
References
Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, et al. Latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J. 2009;33:956–73.
American Thoracic Society, Centers for Disease Control and Prevention. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–95.
Dick M, Madhukar P, George C. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection—areas of uncertainty and recommendations for research. Ann Intern Med. 2007;146:340–354.
Royal College of Physicians. Tuberculosis: national clinical guidelines for diagnosis, management, prevention, and control. London: Royal College of Physicians; 2006. http://www.nice.org.uk.
Wang L, Turner MO, Elwood RK, Schulzer M, Fitzgerald JM. A meta-analysis of the effect of Bacille Calmette Guerin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804–9.
CDC. Guide for primary health care providers: targeted tuberculin testing and treatment of latent tuberculosis infection. Department of Health and Human Services Centers for Disease Control and Prevention National Center for HIV, STD, and TB Prevention Division of Tuberculosis Elimination-Atlanta, Georgia 2005.
Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immunebased diagnosis of tuberculosis. Lancet. 2000;356:1099–104.
MMWR. Guidelines for using the QuantiFERON®-TB gold test for detecting mycobacterium tuberculosis infection, United States Recommendations and Reports December 16. 2005;54:49–55.
Arend SM, Engelhard AC, Groot G, De Boer K, Andersen P, Ottenhoff TH, et al. Tuberculin skin testing compared with T-cell responses to Mycobacterium tuberculosis-specific and nonspecific antigens for detection of latent infection in persons with recent tuberculosis contact. Clin Diagn Lab Immunol. 2001;8:1089–96.
Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med. 2004;170:65–9.
Ravn P, Munk ME, Andersen AB, Lundgren B, Lundgren JD, Nielsen LN, et al. Prospective evaluation of a whole-blood test using Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10 for diagnosis of active tuberculosis. Clin Diagn Lab Immunol. 2005;12:491–6.
Ferrara G, Losi M, Meacci M, Meccugni B, Piro R, Roversi P, et al. Routine hospital use of a new commercial whole blood interferon-g assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2005;172:631–5.
Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005;293:2756–61.
Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, et al. Specific detection of tuberculosis infection: an interferon-g-based assay using new antigens. Am J Respir Crit Care Med. 2004;170:59–64.
CDC. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR 2000;49:1–54.
Tuuminen T, Sorva S, Liippo K, Vasankari T, Soini H, Eriksén-Neuman B, et al. Feasibility of commercial interferon-gamma-based methods for the diagnosis of latent Mycobacterium tuberculosis infection in Finland, a country of low incidence and high bacille Calmette-Guérin vaccination coverage. Clin Microbiol Infect. 2007;13:836–8.
Kobashi Y, Obase Y, Fukuda M, Yoshida K, Miyashita N, Fujii M. Oka M: Usefulness of QuantiFERON TB-2G, a diagnostic method for latent tuberculosis infection, in a contact investigation of health care workers. Intern Med. 2007;46:1543–9.
Mazurek GH, Weis SE, Moonan PK, Daley CL, Bernardo J, Lardizabal AA, et al. Prospective comparison of the tuberculin skin test and 2 whole-blood interferon-gamma release assays in persons with suspected tuberculosis. Clin Infect Dis. 2007;45:837–45.
Kang YA, Lee HW, Hwang SS, Um SW, Han SK, Shim YS, et al. Usefulness of whole-blood interferon-gamma assay and interferon-gamma enzyme-linked immunospot assay in the diagnosis of active pulmonary tuberculosis. Chest. 2007;132:959–65.
Detjen AK, Keil T, Roll S, Hauer B, Mauch H, Wahn U, et al. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin Infect Dis. 2007;45:322–8.
Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, et al. Depressed T-cell interferon-g responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999;180:2069–73.
Mazurek GH, Zajdowicz MJ, Hankinson AL, Costigan DJ, Toney SR, Rothel JS, et al. Detection of Mycobacterium tuberculosis infection in United States Navy recruits using the tuberculin skin test or whole-blood interferon-gamma release assays. Clin Infect Dis. 2007;45:826–36.
Jones S, de Gijsel D, Wallach FR, Gurtman AC, Shi Q, Sacks H. Utility of QuantiFERON-TB Gold in-tube testing for latent TB infection in HIV-infected individuals. Int J Tuberc Lung Dis. 2007;11:1190–5.
Grimes CZ, Hwang LY, Williams ML, Austin CM, Graviss EA. Tuberculosis infection in drug users: interferon-gamma release assay performance. Int J Tuberc Lung Dis. 2007;11:1183–9.
Takahashi H, Shigehara K, Yamamoto M, Suzuki C, Naishiro Y, Tamura Y, et al. Interferon gamma assay for detecting latent tuberculosis infection in rheumatoid arthritis patients during infliximab administration. Rheumatol Int. 2007;27:1143–8.
Franken WP, Timmermans JF, Prins C, Slootman EJ, Dreverman J, Bruins H, et al. Comparison of mantoux and QuantiFERON TB gold tests for diagnosis of latent tuberculosis infection in army personnel. Clin Vaccine Immunol. 2007;14:477–80.
Stephan C, Wolf T, Goetsch U, et al. Comparing QuantiFERON-tuberculosis gold, T-SPOT tuberculosis and tuberculin skin test in HIV-infected individuals from a low prevalence tuberculosis country. AIDS. 2008;22:2471–9.
WHO: Addressing poverty in TB control options for national TB control programmes. who/htm/tb/2005.352.
Carvalho AC, Pezzoli MC, El-Hamad I, Arce P, Bigoni S, Scarcella C, Indelicato AM, Scolari C, Carosi G, Matteelli A. QuantiFERON-TB Gold test in the identification of latent tuberculosis infection in immigrants. J Infect. 2007;55:164–8
Broekmans JF, Migliori GB, Rieder HL, Lees J, Ruutu P, Loddenkemper R, et al. World Health Organization, International Union Against Tuberculosis and Lung Disease, and Royal Netherlands Tuberculosis Association Working Group. European framework for tuberculosis control and elimination in countries with a low incidence. Recommendations of the World Health Organization (WHO), International Union Against Tuberculosis and Lung Disease (IUATLD) and Royal Netherlands Tuberculosis Association (KNCV) Working Group. Eur Respir J. 2002;19:765–75.
Ministero della Salute: Linee-guida per il controllo della malattia tubercolare, su proposta del Ministro della Sanità, ai sensi dell’art. 115, comma 1, lettera b), del decreto legislativo 31 marzo 1998, n. 112.
WHO: DOTS expansion plan to stop TB in the WHO European regions 2002–2006. ISBN 92 890 1367 2, World Health Organization 2002.
http://www.ministerosalute.it/imgs/C_17_pubblicazioni_613_allegato.pdf.
Diel R, Loddenkemper R, Meywald-Walter K, Niemann S, Nienhaus A. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2008;177:1164–70
Soysal A, Torun T, Efe S, Gencer H, Tahaoglu K, Bakir M. Evaluation of cut-off values of interferon-gamma-based assays in the diagnosis of M. tuberculosis infection. Int J Tuberc Lung Dis. 2008;12:50–6.
Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192–204.
WHO: Selected vaccine introduction status into routine immunization. Selected vaccine introduction status into routine infant immunization worldwide, 2003. www.who.int/entity/immunization_monitoring/routine/schedule_analysis_2003.pdf.
Bocchino M, Matarese A, Bellofiore B, Giacomelli P, Santoro G, Balato N, Castiglione F, Scarpa R, Perna F, Signoriello G, Galati D, Ponticiello A, Sanduzzi A. Performance of two commercial blood IFN-gamma release assays for the detection of Mycobacterium tuberculosis infection in patient candidates for anti-TNF-alpha treatment. Eur J Clin Microbiol Infect Dis. 2008 May 10.
Ponce de Leon JD, Acevedo-Vasquez E, Alvizuri S, Gutierrez C, Cucho M, Alfaro J, Perich R, Sanchez-Torres A, Pastor C, Sanchez-Schwartz C, Medina M, Gamboa R, Ugarte M: Comparison of an interferon-gamma assay with tuberculin skin testing for detection of tuberculosis (TB) Infection in Patients with Rheumatoid Arthritis in a TB-Endemic Population. J Rheumatol. 2008 May;35:776–81.
Karam F, Mbow F, Fletcher H, Senghor CS, Coulibaly KD, LeFevre AM, Ngom Gueye NF, Dieye T, Sow PS, Mboup S, Lienhardt C. Sensitivity of IFN-gamma release assay to detect latent tuberculosis infection is retained in HIV-infected patients but dependent on HIV/AIDS progression. PLoS One. 2008;3:e1441.
Rangaka MX, Wilkinson KA, Seldon R, Van Cutsem G, Meintjes GA, Morroni C, et al. Effect of HIV-1 infection on T-Cell-based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007;175:514–20. (Epub 2006 Dec 7).
Diel R, Nienhaus A, Loddenkemper R. Cost-effectiveness of interferon-gamma release assay screening for latent tuberculosis infection treatment in Germany. Chest. 2007;131:1424–34.
Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest. 2007;131:1898–906.
Acknowledgments
The authors acknowledge the Provincia di Milano, Assessorato alle Politiche Sociali for the financial support of the study.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orlando, G., Merli, S., Cordier, L. et al. Interferon-γ releasing assay versus tuberculin skin testing for latent tuberculosis infection in targeted screening programs for high risk immigrants. Infection 38, 195–204 (2010). https://doi.org/10.1007/s15010-010-0015-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-010-0015-2