Abstract
Purpose
Intubation of hypoxemic patients is associated with life-threatening adverse events. High-flow therapy by nasal cannula (HFNC) for preoxygenation before intubation has never been assessed by randomized study. Our objective was to evaluate the efficiency of HFNC for preoxygenation, compared to high fraction-inspired oxygen facial mask (HFFM).
Methods
Multicenter, randomized, open-labelled, controlled PREOXYFLOW trial (NCT 01747109) in six French intensive care units. Acute hypoxemic adults requiring intubation were randomly allocated to HFNC or HFFM. Patients were eligible if PaO2/FiO2 ratio was below 300 mmHg, respiratory rate at least 30/min and if they required FiO2 50 % or more to obtain at least 90 % oxygen saturation. HFNC was maintained throughout the procedure, whereas HFFM was removed at the end of general anaesthesia induction. Primary outcome was the lowest saturation throughout intubation procedure. Secondary outcomes included adverse events related to intubation, duration of mechanical ventilation and death.
Results
A total of 124 patients were randomized. In the intent-to-treat analysis, including 119 patients (HFNC n = 62; HFFM n = 57), the median (interquartile range) lowest saturation was 91.5 % (80–96) for HFNC and 89.5 % (81–95) for the HFFM group (p = 0.44). There was no difference for difficult intubation (p = 0.18), intubation difficulty scale, ventilation-free days (p = 0.09), intubation-related adverse events including desaturation <80 % or mortality (p = 0.46).
Conclusions
Compared to HFFM, HFNC as a preoxygenation device did not reduce the lowest level of desaturation.
Similar content being viewed by others
Introduction
Acute respiratory failure is a life-threatening condition in intensive care units (ICU) that often requires ventilator support after endotracheal intubation (ETI). In critically ill patients, ETI carries a higher risk than in elective surgery and can lead to mild to severe complications for 30 % of patients [1]. The most frequently reported complication is desaturation below 80 % (26 %). Severe hypoxemia before ETI has been reported as an independent risk factor of severe desaturation during intubation in ICU [2].
Preoxygenation with a high fraction-inspired oxygen facial mask (HFFM) is recommended to delay arterial desaturation during intubation apnoea [2–4]. The quality of preoxygenation contributes to the safety of the procedure. Nevertheless, it is sometimes ineffective in critically ill patients, especially in hypoxemic patients. Despite well-conducted preoxygenation, desaturation <80 % occurs in a quarter of hypoxemic patients [2]. Non-invasive ventilation (NIV) seems to improve preoxygenation in this population, but these results have not been confirmed in large studies [5].
High-flow therapy by nasal cannula (HFNC) is a new device that provides almost pure oxygen with FiO2 of about 100 %, up to 60 l/min [6]. HFNC seems to generate low positive airway pressure [7, 8], and holding nasal prongs in place after rapid sequence intubation during laryngoscopy could enable operators to perform apnoeic oxygenation and reduce desaturation during ETI [9]. A recent before–after study suggests its ability to improve preoxygenation and to reduce severe hypoxemia during ETI [10].
Although HFNC is widespread, randomized studies have never evaluated this device during preoxygenation. We therefore conducted a multicenter, randomized, controlled trial comparing HFNC and HFFM in preoxygenation before ETI in ICU hypoxemic patients (PREOXYFLOW study). We postulated that HFNC was more efficient than HFFM desaturation during ETI. The secondary objectives were to determine whether HFNC could reduce complications during ETI, and improve morbidity in ICUs.
Methods
Study design, setting, and ethical considerations
PREOXYFLOW is a multicenter, randomized, controlled, parallel, open-label study comparing two preoxygenation strategies for ETI in ICUs. The main objective was to determine whether HFNC was more effective than HFFM in reducing desaturation during ETI after rapid sequence induction.
Patients were enrolled from December 2012 to August 2013 in six French ICUs (three medical, two medical-surgical and one surgical). An independent safety committee oversaw the trial.
Three methods of consent were available. Hypoxemia often prevented the patient from understanding information. Thus, most patients were included after written informed consent was provided by next of kin or an emergency procedure (investigator signature) if next of kin were not immediately available or if there was not enough time before ETI. When available, patients were retrospectively asked for written consent to continue the trial after recovery. Only a few patients were included after self-consent. The ethics committee (“Comité de Protection des Personnes Ouest II” from Angers, France) approved this study protocol on 9 October 2012 (CPP ref. 2012/17).
Randomization
Randomization used fixed blocks of four patients (ratio 1:1) and was stratified by centre. The study statistician generated the allocation list. Patients were allocated to one of the two preoxygenation strategies (HFNC or HFFM) using a secure computer-generated online remote system (Clinisight software) controlled by the independent research promotion unit at the University Hospital of Nantes, which had no role in patient recruitment. Day 1 was the day of inclusion and randomization.
Patients
Eligible patients were adults (18 years or older) with acute hypoxemic respiratory failure, requiring ETI in ICU after rapid sequence induction (prompt general anaesthesia induction and subsequent intubation). Acute hypoxemic respiratory failure was defined as a respiratory rate higher than 30 per minute and a FiO2 requirement of 50 % or more to obtain at least 90 % oxygen saturation, and an estimated PaO2/FiO2 ratio below 300 mmHg, in the 4 h before inclusion.
Exclusion criteria were: contraindication to orotracheal intubation; intubation without anaesthetic rapid sequence induction; ETI for cardiac arrest; asphyxia requiring immediate ETI; nasopharyngeal obstacle; Grade 4 glottis exposure on the Cormack–Lehane scale, adults subject to a legal protection scheme; pregnancy or lack of consent.
Intervention: preoxygenation and intubation procedure
The patients were randomized immediately after inclusion. In the intervention group (HFNC), preoxygenation was performed for 4 min with high-flow nasal cannula (Optiflow™; Fisher & Paykel Healthcare, Auckland, NZ) set to 60 l/min of humidified oxygen flow (FiO2 100 %) [11]. In this group, after induction, the nasal cannulae were maintained in place throughout the ETI, in an attempt to achieve apnoeic oxygenation. In the control group (HFFM), preoxygenation was performed for 4 min with high FiO2 facial mask (15 l/min oxygen flow) [5, 12]. After general anaesthetic induction, the HFFM was removed, enabling laryngoscopy vision.
The patients were intubated according to international recommendations and standard practices at each participating ICU [13]. Patients receiving non-invasive ventilation (NIV), HFFM or HFNC at the time of randomization were switched to their randomly assigned device at the beginning of the preoxygenation procedure. The PREOXYFLOW Study design is summarized in online-only material (see Fig. E1).
Endpoints
The primary outcome was the lowest saturation measured by continuous pulse oximetry (SpO2) during ETI, from the end of rapid sequence induction (beginning of laryngoscopy) to patient connection to mechanical ventilation (see Fig. E1). To improve the quality of data collection, an external observer and the nurse in charge of the patient concurrently assessed this endpoint.
The secondary outcomes were: quality of preoxygenation, including duration, ability of the device to improve SpO2; the quality of the ETI procedure, including adverse events during ETI and in the next hour, difficult intubation rate and intubation difficulty score (IDS); organ failure during the first 5 days (SOFA score); PaO2/FiO2 ratio during the following hour and the first 5 days; morbidity in ICU (time on ventilator, length of stay, ventilation-free days, occurrence of ventilator-associated pneumonia and mortality rate on day 28) [14].
Difficult intubation was defined as three or more laryngoscopies attempts or the need to use alternative devices, or procedure duration longer than 10 min. Adverse events were classified as severe complications (death, cardiac arrest, desaturation <80 %; severe collapse defined by systolic blood pressure <80 or vasopressor introduction or increasing doses more than 30 %) or mild to moderate complications (severe ventricular or supraventricular arrhythmia requiring intervention, oesophageal intubation, dangerous agitation with Richmond agitation scale >3, vomiting with aspiration of gastric content, dental injury). All patients were followed until discharge from ICU and up to day 28. An independent monitoring board analyzed the data.
Sample size
The primary outcome was the lowest SpO2 during ETI up to connection to mechanical ventilation. The trial was designed to detect an increase of 6 % in SpO2 from 85 % in the HFFM group to 91 % in the HFNC group. As no data were available, this hypothesis was based on previous results comparing NIV to HFFM [5]. With 80 % power, a 5 % type I error (two-sided) and 2 % attrition rate, the required sample size was 122 patients. A few weeks after the beginning of the study, two patients had to be excluded on the basis of exclusion criteria. Thus, the independent steering committee decided to increase the sample size to 124 in order to preserve the power of the study.
Statistical analysis
Analyses were performed in an intent-to-treat population including all the randomized patients except two patients who withdrew their consent, two patients who did not meet the inclusion criteria and one patient who improved before ETI.
Baseline characteristics were described for each group. Continuous variables were presented with mean and standard deviation, or median and interquartile range, when the assumption of normality was not met. Categorical data were expressed as numbers and percentages.
The primary outcome was compared between the two groups using the Van Elteren test (non-parametric test taking randomisation stratified by centre into account). Sensitivity analyses were performed a posteriori: the main criterion was lacking for one patient in the HFFM group, so analysis with best-case imputation was performed. Analysis was completed by four sensitivity tests with adjustment to baseline saturation or baseline PaO2/FiO2, to SpO2 at the beginning of preoxygenation or SpO2 at the end of preoxygenation.
The secondary outcomes were compared between groups with generalised linear mixed models (logistic regression) or nonparametric Van Elteren Tests for the quantitative outcome, to take stratification into account. For SpO2 at the end of preoxygenation, an adjustment was made on SpO2 at the beginning of preoxygenation. The proportions of patients with severe and moderate complications in the two groups were compared using the Chi square or Fisher tests.
Statistical tests were two-sided, with a statistical significance of p < 0.05. SAS software v.9.3® was used for all statistical analyses.
Results
Patients
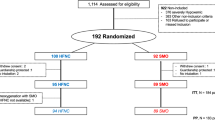
Between December 2012 and August 2013, 2936 patients were admitted. Out of 482 patients who underwent ETI on admission or during their stay in the ICU, 124 with hypoxemic respiratory failure were included for randomization in the PREOXYFLOW study (Fig. 1). Five patients were excluded (two withdrew consent, two major inclusion criteria deviations, one patient improved before ETI and was not intubated). Finally, 119 patients were included for the ITT analysis (62 in the HFNC group and 57 in the HFFM group). One patient in HFNC underwent ETI without preoxygenation due to extreme emergency.
The main baseline characteristics were similar between the two groups (Table 1). Infectious pneumonia was the main cause of respiratory failure on admission with 40.3 % in HFNC and 50.9 % in HFFM. At inclusion, the mean (SD) PaO2/FiO2 ratio was 120.2 (55.7) mmHg in HFNC and 115.7 (63) mmHg in the HFFM.
Airways and ETI are described in Table 2. Difficult facial mask ventilation and difficult intubation criteria were quite similar in the two groups.
Primary outcome: lowest oxygen saturation during the ETI Procedure
In the ITT analysis (Table 3), there was no statistical difference regarding the lowest recorded SpO2 during ETI comparing the HFNC group median (IQR), 91.5 % (80–96), versus the HFFM group, 89.5 % (81–95), p = 0.44. Both groups exhibited wide variability in lowest SpO2 values (Fig. 2). As data were missing for one patient for the main criteria in HFFM group, a sensitivity analysis was performed with best-case imputation (SpO2 = 100 %), with no difference between the two groups (p = 0.55). Considering the influence of the severity of acute respiratory failure, analyses were carried out with four other a posteriori sensitivity tests, with adjustment on PaO2/FiO2 on inclusion, SpO2 on inclusion, and SpO2 at the beginning and end of the preoxygenation phase. None of these analyses found any difference between HFNC and HFFM (p = 0.85, p = 0.60, p = 0.49 and p = 0.79, respectively).
Secondary outcomes
The secondary outcomes are reported in Table 3.
Oxygenation during preoxygenation
The 4-min preoxygenation phase was completed in 58 (93.5 %) patients in HFNC and 54 (94.7 %) in HFFM (p = 0.83). Along preoxygenation, mean (SD) SpO2 rose slightly from 95.4 (3.9) to 97.1 (3.8) in the HFNC group and from 94.1 (6) to 96.3 (4.4) in the HFFM group with no difference in the delta SpO2 (p = 0.98). The duration of preoxygenation did not influence the lowest SpO2 during ETI (see Table E1). Preoxygenation did not succeed in raising SpO2 above 90 % (p = 0.49) in only four patients (6.5 %) in HFNC and 2 (3.5 %) in HFFM.
Description of the ETI procedure
All randomized patients were successfully intubated. Maintaining the HFNC in place did not increase the rate of difficult intubation: 1.6 and 7.1 %, respectively, in HFNC and HFFM (p = 0.18). The median (IQR) IDS score was similar 3 (2–4) in both groups (p = 0.63).
Outcome in ICUs
Patients in HFNC saw a significantly shorter median (IQR) duration of mechanical ventilation of 6 (4–14) days than those in HFFM of 10 (5–17) days (p = 0.02). However, there was no difference on day 28 of ventilator-free days (p = 0.09), SOFA score during the first 5 days, length of ICU stay or ventilator-associated pneumonia. The mortality rate on day 28 was 35.4 and 42.1 % in HFNC and HFFM (p = 0.46), respectively.
Complications during the ETI procedure and the following hour (Table 4)
At least one complication occurred in 36 (58.1 %) and 39 (68.4 %) patients in HFNC and HFFM (p = 0.24), respectively. At least one severe complication occurred in 36 (58.1 %) patients in HFNC and 38 (66.7 %) patients in HFFM (p = 0.33). Severe desaturation (SpO2 < 80 %) was the main severe complication, with 16 (25.8 %) patients in HFNC and 13 (22.3 %) in HFFM (p = 0.70). Only one cardiac arrest was observed in the whole population during ETI. No patient died during ETI or over the next hour. No injury or unexpected effect related to the HFNC device was observed.
Discussion
The aim of the study was to compare HFNC preoxygenation to HFFM for intubation in severely hypoxemic patients. Using HFNC without discontinuation during an apnoeic period was not more effective in preventing desaturation, regardless of the severity of respiratory distress. Our results also confirm that ETI for hypoxemic patients in ICUs is risky, with more than 50 % of serious adverse events and 25 % of desaturation below 80 % irrespective of the preoxygenation device, despite low difficult intubation rate [2]. This trial focusing on the ETI procedure highlighted no difference regarding outcome in ICU.
This is the first clinical trial comparing HFNC and HFFM as preoxygenation devices in a randomised multicentre controlled design. It matches medical and surgical patients with experienced staff. Despite the hypoxemia, the emergency and risk of ETI, we succeeded in including 119 patients in six centres over a 9-month period.
Our results are in contradiction with a single centre trial, before–after design included incidental patients regardless of the reasons for intubation. For most of them, respiratory failure was not the main issue. This probably explains the difference in favour of HFNC [10].
HFNC exhibits several theoretical advantages. Firstly, this device was reported to improve oxygenation through high-flow oxygen and to create positive end expiratory pressure (PEEP) [15, 16]. Secondly, compared to a low-flow oxygen device, HFNC significantly increases the PaO2/FiO2 ratio and end expiratory lung volume in hypoxemic patients [17]. It may minimize desaturation during ETI. Thirdly, contrary to HFFM, HFNC can be maintained in place during ETI without restricting glottis catheter insertion. Considering that high oxygen flow may keep upper airways open, HFNC could lead to apnoeic oxygenation [7, 9, 18].
In our study, despite these theoretical advantages, HFNC did not seem to be more efficient than HFFM in preventing desaturation. Several reasons may explain these results. Our study included patients with severe hypoxemia (mean PaO2/FiO2 about 120 mmHg), and most of them suffered from pneumonia. HFNC only creates limited levels of PEEP [7, 19]. Non-invasive ventilation (NIV) might be an interesting alternative with higher PEEP levels. However, effects on functional respiratory capacity, especially during a 4-min preoxygenation, are unpredictable [20]. Extending preoxygenation for more than 4 min is not a better strategy either [11, 21]. Regarding apnoeic oxygenation, nasopharyngeal oxygen insufflation during ETI has been reported to improve the duration of apnoea without desaturation, only in studies with a small number of patients [9, 18]. Controversially, after general anaesthesia, as in obese or sleep apnoea patients, upper airway collapsing pressures dramatically increase [22–25]. This probably explains why HFNC failed to achieve apnoeic oxygenation during ETI.
Despite well-conducted randomization, the population at baseline in the two groups exhibited slight differences especially regarding difficult airway. Subgroups or multivariate analyses did not detect any influence on the primary outcome (data not shown).
The choice of the primary outcome can be discussed. We decided to consider minimal saturation instead of desaturations below 80 % in order to detect hypoxemic events regardless of their severity. Pulse oximetry is not as relevant and accurate as measured arterial oxygen saturation (SaO2) or partial oxygen pressure (PaO2) [26, 27]. Nevertheless, it is the only non-invasive, easily bedside-monitored criterion during everyday emergencies in ICU. It informs about apnoea tolerance and warns when to interrupt ETI. PaO2 in an arterial blood gases test would have been a more reliable evaluation of blood oxygenation during and immediately after an ETI procedure. This would have required systematic catheterization to establish an indwelling arterial line, which seemed unthinkable due to the severity of the hypoxemia and level of ETI emergency. Moreover, given the very rapid variation, a research nurse was specifically dedicated to monitor SpO2 throughout the procedure to improve data collection.
These negative results have to be balanced by power issues. Sample size calculation was based on reasonable hypothesis but settled by a single preliminary study [5]. We concede that our study is not an extensive trial but reflects pragmatic issues of ETI performed by trained operators in ICU. Further studies are needed to confirm these results in hypoxemic patients. The choice of the conventional group could also be argued. A preliminary study assessed the usefulness of NIV in preoxygenation, but to our knowledge no randomized multicentre study has confirmed this hypothesis [5]. As a result, whether NIV or HFFM was the best control group remains unsure. Considering the advantages reported, the contribution of video laryngoscopy in ETI algorithm in ICU also has to be assessed [28–30]. Finally, preoxygenation quality has to be a priority area for improvement but should always be implemented in an intubation management protocol [31].
HFNC was unable to prevent desaturation but provided similar lowest saturation levels to those achieved with HFFM. Therefore, this device could be considered as a safe alternative to HFFM for preoxygenation in ICUs with specific equipment and when used by trained staff. The timing of invasive mechanical ventilation probably governs the depth of desaturation during ETI more than the preoxygenation device.
Conclusion
Despite the theoretical advantages, in acute severely hypoxemic patients, high flow nasal cannula oxygen therapy as a preoxygenation device was not more efficient than a high FiO2 flow face mask in preventing desaturation during ETI. This study also confirms that ETI remains a landmark in a hypoxemic patient’s history, with more than 50 % of severe complications.
References
De Jong A, Molinari N, Terzi N, Mongardon N, Arnal JM, Guitton C, Allaouchiche B, Paugam-Burtz C, Constantin JM, Lefrant JY, Leone M, Papazian L, Asehnoune K, Maziers N, Azoulay E, Pradel G, Jung B, Jaber S (2013) Early identification of patients at risk for difficult intubation in the intensive care unit: development and validation of the MACOCHA score in a multicenter cohort study. Am J Respir Crit Care Med 187:832–839
Jaber S, Amraoui J, Lefrant JY, Arich C, Cohendy R, Landreau L, Calvet Y, Capdevila X, Mahamat A, Eledjam JJ (2006) Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Crit Care Med 34:2355–2361
Baraka A (2009) Preoxygenation of the morbidly obese with obstructive sleep apnea. Middle East J Anesthesiol 20:141–142
Benumof JL (1999) Preoxygenation: best method for both efficacy and efficiency. Anesthesiology 91:603–605
Baillard C, Fosse JP, Sebbane M, Chanques G, Vincent F, Courouble P, Cohen Y, Eledjam JJ, Adnet F, Jaber S (2006) Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med 174:171–177
Roca O, Riera J, Torres F, Masclans JR (2010) High-flow oxygen therapy in acute respiratory failure. Respir Care 55:408–413
Parke R, McGuinness S, Eccleston M (2009) Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth 103:886–890
Wettstein RB (2013) A fresh look at the physiologic effects of high-flow nasal cannulae and the role they play in patient care. Respir Care 58:715–716
Teller LE, Alexander CM, Frumin MJ, Gross JB (1988) Pharyngeal insufflation of oxygen prevents arterial desaturation during apnea. Anesthesiology 69:980–982
Miguel-Montanes R, Hajage D, Messika J, Bertrand F, Gaudry S, Rafat C, Labbe V, Dufour N, Jean-Baptiste S, Bedet A, Dreyfuss D, Ricard JD, (2014) Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med (in press)
Mort TC (2005) Preoxygenation in critically ill patients requiring emergency tracheal intubation. Crit Care Med 33:2672–2675
Apfelbaum JL, Hagberg CA, Caplan RA, Blitt CD, Connis RT, Nickinovich DG, Hagberg CA, Caplan RA, Benumof JL, Berry FA, Blitt CD, Bode RH, Cheney FW, Connis RT, Guidry OF, Nickinovich DG, Ovassapian A (2013) American Society of Anesthesiologists Task Force on Management of the Difficult Airway Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 118:251–270
Walz JM, Zayaruzny M, Heard SO (2007) Airway management in critical illness. Chest 131:608–620
Adnet F, Borron SW, Racine SX, Clemessy JL, Fournier JL, Plaisance P, Lapandry C (1997) The intubation difficulty scale (IDS): proposal and evaluation of a new score characterizing the complexity of endotracheal intubation. Anesthesiology 87:1290–1297
Groves N, Tobin A (2007) High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care 20:126–131
Parke RL, McGuinness SP, Eccleston ML (2011) A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care 56:265–270
Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF (2011) Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth 107:998–1004
Ramachandran SK, Cosnowski A, Shanks A, Turner CR (2010) Apneic oxygenation during prolonged laryngoscopy in obese patients: a randomized, controlled trial of nasal oxygen administration. J Clin Anesth 22:164–168
Parke RL, McGuinness SP (2013) Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care 58:1621–1624
Dellamonica J, Lerolle N, Sargentini C, Beduneau G, Di Marco F, Mercat A, Richard JC, Diehl JL, Mancebo J, Rouby JJ, Lu Q, Bernardin G, Brochard L (2011) PEEP-induced changes in lung volume in acute respiratory distress syndrome. Two methods to estimate alveolar recruitment. Intensive Care Med 37:1595–1604
Mort TC, Waberski BH, Clive J (2009) Extending the preoxygenation period from 4 to 8 min in critically ill patients undergoing emergency intubation. Crit Care Med 37:68–71
Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR (2005) Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology 103:470–477
Eastwood PR, Szollosi I, Platt PR, Hillman DR (2002) Collapsibility of the upper airway during anesthesia with isoflurane. Anesthesiology 97:786–793
Eastwood PR, Szollosi I, Platt PR, Hillman DR (2002) Comparison of upper airway collapse during general anaesthesia and sleep. Lancet 359:1207–1209
Kirkness JP, Peterson LA, Squier SB, McGinley BM, Schneider H, Meyer A, Schwartz AR, Smith PL, Patil SP (2011) Performance characteristics of upper airway critical collapsing pressure measurements during sleep. Sleep 34:459–467
Perkins GD, McAuley DF, Giles S, Routledge H, Gao F (2003) Do changes in pulse oximeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit Care 7:R67
Seguin P, Le Rouzo A, Tanguy M, Guillou YM, Feuillu A, Malledant Y (2000) Evidence for the need of bedside accuracy of pulse oximetry in an intensive care unit. Crit Care Med 28:703–706
De Jong A, Clavieras N, Conseil M, Coisel Y, Moury PH, Pouzeratte Y, Cisse M, Belafia F, Jung B, Chanques G, Molinari N, Jaber S (2013) Implementation of a combo videolaryngoscope for intubation in critically ill patients: a before-after comparative study. Intensive Care Med 39:2144–2152
De Jong A, Molinari N, Conseil M, Coisel Y, Pouzeratte Y, Belafia F, Jung B, Chanques G, Jaber S (2014) Video laryngoscopy versus direct laryngoscopy for orotracheal intubation in the intensive care unit: a systematic review and meta-analysis. Intensive Care Med 40:629–639
Mosier JM, Law JA (2014) Airway management in the critically ill. Intensive Care Med 40:727–729
Jaber S, Jung B, Corne P, Sebbane M, Muller L, Chanques G, Verzilli D, Jonquet O, Eledjam JJ, Lefrant JY (2010) An intervention to decrease complications related to endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Intensive Care Med 36:248–255
Acknowledgments
Contributors: We thank Yohann FOUCHER, EA4275 Biostatistician at Nantes University Hospital, for assistance in designing the study. We are grateful to all medical staff, nurses, and research staff at the six sites for inclusion and data collection. We thank Monique MARGUERITE for administrative and logistic support and Marion RIGOT for creating the electronic case report form; and Dr Anne Chiffoleau, MD for safety monitoring. Financial and material support for the research and grant, funding and provision of equipment and supplies: This study was supported by the French Ministry of Health (Interregional French Clinical Hospital Research Program grant (PHRCi 2012– API12/N/077) in addition to a grant for research & innovation missions allocated to the university hospital of Nantes by Fisher & Paykel Healthcare. Nantes University Hospital sponsored the study. Fisher & Paykel participated for 14 % of the total budget including supply of consumables to the six participating centres. Fisher & Paykel did not participate in the study design or in data collection, analysis and interpretation of the data, or in the writing, review, approval and decision to submit the manuscript for publication.
Conflicts of interest
Dr. Christophe Guitton and Dr. Mickael Vourc’h had full access to all of the data in the study and take complete responsibility for the integrity of the data and the accuracy of the data analysis. No conflict of interest: Mickaël Vourc’h, Christelle Volteau, Konstantinos Bachoumas, Noëmie Clavieras, Pierre-Yves Egreteau, Jean Reignier, Gwenaël Prat, Noëlle Brule, Daniel Villers, Cedric Bretonniere, Christophe Guitton. Pr Asfar received consulting fees from LFB. Pr Jaber received consulting fees from Dräger, Hamilton, Maquet and Fisher Paykel. Pr Mercat received grant support (clinical research) from Covidien, Maquet and General Electric and personal fees from Faron Pharmaceuticals (member of steering committee), Air Liquide Medical Systems and Covidien. Pr Asehnoune served as board member for Astellas, received grant support from Astellas and Pfizer; lectured for B-Braun and Fresenius. Dr Roquilly reports conflict of interest with Merck laboratory (investigator in a study).
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: Intubation in hypoxemic patients is a high-risk event. Preoxygenation contributes to the safety of the procedure. Strategies to decrease deep desaturation during intubation in hypoxemic patients have been little studied. Despite theoretical advantages and widespread use, randomized studies have never evaluated high-flow therapy in preoxygenation before intubation for hypoxemic patients. This study shows that this new device, maintained in place from the beginning of preoxygenation to the end of the intubation procedure, does not prevent deep desaturation during intubation in severely hypoxemic patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vourc’h, M., Asfar, P., Volteau, C. et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med 41, 1538–1548 (2015). https://doi.org/10.1007/s00134-015-3796-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-3796-z