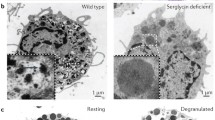
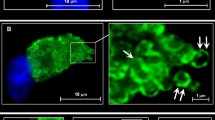
Abstract
Mast cells store an impressive array of preformed compounds (mediators) in their secretory granules. When mast cells degranulate, these are released and have a profound impact on any condition in which mast cell degranulation occurs. The preformed mast cell mediators include well-known substances such as histamine, proteoglycans, proteases, and preformed cytokines, as well as several recently identified compounds. Mast cells have recently been implicated in a large number of novel pathological settings in addition to their well-established contribution to allergic reactions, and there is consequently a large current interest in the molecular mechanisms by which mast cells act in the context of a given condition. In many cases, preformed mast cell mediators have been shown to account for functions ascribed to mast cells, and these compounds are hence emerging as major players in numerous pathologies. In this review we summarize the current knowledge of preformed mast cell mediators.


Similar content being viewed by others
References
Kalesnikoff J, Galli SJ (2008) New developments in mast cell biology. Nat Immunol 9:1215–1223
Marshall JS (2004) Mast-cell responses to pathogens. Nat Rev Immunol 4:787–799
Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ (2006) Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 442:997–1002
Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ (2007) Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol 8:1095–1104
Blank U, Rivera J (2004) The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol 25:266–273
Turner H, Kinet JP (1999) Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature 402:B24–B30
Rivera J, Fierro NA, Olivera A, Suzuki R (2008) New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol 98:85–120
Galli SJ, Nakae S, Tsai M (2005) Mast cells in the development of adaptive immune responses. Nat Immunol 6:135–142
Boyce JA (2007) Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev 217:168–185
Riley JF (1953) Histamine in tissue mast cells. Science 118:332
Rothschild AM, Schayer RW (1959) Characterization of histidine decarboxylase from rat peritoneal fluid mast cells. Biochim Biophys Acta 34:392–398
Ringvall M, Rönnberg E, Wernersson S, Duelli A, Henningsson F, Åbrink M, Garcia-Faroldi G, Fajardo I, Pejler G (2008) Serotonin and histamine storage in mast cell secretory granules is dependent on serglycin proteoglycan. J Allergy Clin Immunol 121:1020–1026
Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, Sakurai E, Buzas E, Kovacs P, Csaba G, Kittel A, Okada M, Hara M, Mar L, Numayama-Tsuruta K, Ishigaki-Suzuki S, Ohuchi K, Ichikawa A, Falus A, Watanabe T, Nagy A (2001) Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett 502:53–56
Koarai A, Ichinose M, Ishigaki-Suzuki S, Yamagata S, Sugiura H, Sakurai E, Makabe-Kobayashi Y, Kuramasu A, Watanabe T, Shirato K, Hattori T, Ohtsu H (2003) Disruption of l-histidine decarboxylase reduces airway eosinophilia but not hyperresponsiveness. Am J Respir Crit Care Med 167:758–763
Makabe-Kobayashi Y, Hori Y, Adachi T, Ishigaki-Suzuki S, Kikuchi Y, Kagaya Y, Shirato K, Nagy A, Ujike A, Takai T, Watanabe T, Ohtsu H (2002) The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J Allergy Clin Immunol 110:298–303
Sasaguri Y, Wang KY, Tanimoto A, Tsutsui M, Ueno H, Murata Y, Kohno Y, Yamada S, Ohtsu H (2005) Role of histamine produced by bone marrow-derived vascular cells in pathogenesis of atherosclerosis. Circ Res 96:974–981
Musio S, Gallo B, Scabeni S, Lapilla M, Poliani PL, Matarese G, Ohtsu H, Galli SJ, Mantegazza R, Steinman L, Pedotti R (2006) A key regulatory role for histamine in experimental autoimmune encephalomyelitis: disease exacerbation in histidine decarboxylase-deficient mice. J Immunol 176:17–26
Ohtsu H (2008) Progress in allergy signal research on mast cells: the role of histamine in immunological and cardiovascular disease and the transporting system of histamine in the cell. J Pharmacol Sci 106:347–353
Benditt EP, Wong RL, Arase M, Roeper E (1955) 5-Hydroxytryptamine in mast cells. Proc Soc Exp Biol Med 90:303–304
Sjoerdsma A, Waalkes TP, Weissbach H (1957) Serotonin and histamine in mast cells. Science 125:1202–1203
Morishima T (1970) 5-Hydroxytryptamine (serotonin) and 5-hydroxytryptophan in mast cells of human mastocytosis. Tohoku J Exp Med 102:121–126
Kushnir-Sukhov NM, Brown JM, Wu Y, Kirshenbaum A, Metcalfe DD (2007) Human mast cells are capable of serotonin synthesis and release. J Allergy Clin Immunol 119:498–499
Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M (2009) Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci USA 106:10332–10337
Freeman JG, Ryan JJ, Shelburne CP, Bailey DP, Bouton LA, Narasimhachari N, Domen J, Simeon N, Couderc F, Stewart JK (2001) Catecholamines in murine bone marrow derived mast cells. J Neuroimmunol 119:231–238
Kanerva K, Lappalainen J, Makitie LT, Virolainen S, Kovanen PT, Andersson LC (2009) Expression of antizyme inhibitor 2 in mast cells and role of polyamines as selective regulators of serotonin secretion. PLoS One 4:e6858
Tiwari N, Wang CC, Brochetta C, Ke G, Vita F, Qi Z, Rivera J, Soranzo MR, Zabucchi G, Hong W, Blank U (2008) VAMP-8 segregates mast cell-preformed mediator exocytosis from cytokine trafficking pathways. Blood 111:3665–3674
Puri N, Roche PA (2008) Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc Natl Acad Sci USA 105:2580–2585
Blott EJ, Griffiths GM (2002) Secretory lysosomes. Nat Rev Mol Cell Biol 3:122–131
Schwartz LB, Austen KF (1980) Enzymes of the mast cell granule. J Invest Dermatol 74:349–353
Schwartz LB, Lewis RA, Seldin D, Austen KF (1981) Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J Immunol 126:1290–1294
Wolters PJ, Laig-Webster M, Caughey GH (2000) Dipeptidyl peptidase I cleaves matrix-associated proteins and is expressed mainly by mast cells in normal dog airways. Am J Respir Cell Mol Biol 22:183–190
Dragonetti A, Baldassarre M, Castino R, Demoz M, Luini A, Buccione R, Isidoro C (2000) The lysosomal protease cathepsin D is efficiently sorted to and secreted from regulated secretory compartments in the rat basophilic/mast cell line RBL. J Cell Sci 113(Pt 18):3289–3298
Henningsson F, Yamamoto K, Saftig P, Reinheckel T, Peters C, Knight SD, Pejler G (2005) A role for cathepsin E in the processing of mast-cell carboxypeptidase A. J Cell Sci 118:2035–2042
Turk V, Turk B, Turk D (2001) Lysosomal cysteine proteases: facts and opportunities. EMBO J 20:4629–4633
Yurt RW, Leid RW Jr, Austen KF (1977) Native heparin from rat peritoneal mast cells. J Biol Chem 252:518–521
Enerback L, Kolset SO, Kusche M, Hjerpe A, Lindahl U (1985) Glycosaminoglycans in rat mucosal mast cells. Biochem J 227:661–668
Metcalfe DD, Lewis RA, Silbert JE, Rosenberg RD, Wasserman SI, Austen KF (1979) Isolation and characterization of heparin from human lung. J Clin Invest 64:1537–1543
Thompson HL, Schulman ES, Metcalfe DD (1988) Identification of chondroitin sulfate E in human lung mast cells. J Immunol 140:2708–2713
Pejler G, Abrink M, Wernersson S (2009) Serglycin proteoglycan: regulating the storage and activities of hematopoietic proteases. Biofactors 35:61–68
Duelli A, Rönnberg E, Waern I, Ringvall M, Kolset SO, Pejler G (2009) Mast cell differentiation and activation is closely linked to expression of genes coding for the serglycin proteoglycan core protein and a distinct set of chondroitin sulfate and heparin sulfotransferases. J Immunol 183:7073–7083
Åbrink M, Grujic M, Pejler G (2004) Serglycin is essential for maturation of mast cell secretory granule. J Biol Chem 279:40897–40905
Enerback L (1974) Berberine sulphate binding to mast cell polyanions: a cytofluorometric method for the quantitation of heparin. Histochemistry 42:301–313
Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, Geissler EN, Galli SJ (1991) Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci USA 88:6382–6386
Schwartz LB, Irani AM, Roller K, Castells MC, Schechter NM (1987) Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol 138:2611–2615
Pejler G, Åbrink M, Ringvall M, Wernersson S (2007) Mast cell proteases. Adv Immunol 95:167–255
Pejler G, Ronnberg E, Waern I, Wernersson S (2010) Mast cell proteases: multifaceted regulators of inflammatory disease. Blood 115:4981–4990
McNeil HP, Shin K, Campbell IK, Wicks IP, Adachi R, Lee DM, Stevens RL (2008) The mouse mast cell-restricted tetramer-forming tryptases mouse mast cell protease 6 and mouse mast cell protease 7 are critical mediators in inflammatory arthritis. Arthritis Rheum 58:2338–2346
Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, Adachi R, Gurish MF, Gobezie R, Stevens RL, Lee DM (2009) Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol 182:647–656
Waern I, Jonasson S, Hjoberg J, Bucht A, Abrink M, Pejler G, Wernersson S (2009) Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J Immunol 183:6369–6376
Sun J, Zhang J, Lindholt JS, Sukhova GK, Liu J, He A, Abrink M, Pejler G, Stevens RL, Thompson RW, Ennis TL, Gurish MF, Libby P, Shi GP (2009) Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation 120:973–982
Scandiuzzi L, Beghdadi W, Daugas E, Åbrink M, Tiwari N, Brochetta C, Claver J, Arouche N, Zang X, Pretolani M, Monteiro RC, Pejler G, Blank U (2010) Murine mast cell MCP4 chymase deteriorates renal function by contributing to inflammation and fibrosis in immune complex-mediated glomerulonephritis. J Immunol 185:624–633
Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R (2007) The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem 282:20809–20815
Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM (2008) Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol 180:4885–4891
Schechter NM, Irani AM, Sprows JL, Abernethy J, Wintroub B, Schwartz LB (1990) Identification of a cathepsin G-like proteinase in the MCTC type of human mast cell. J Immunol 145:2652–2661
Schechter NM, Wang ZM, Blacher RW, Lessin SR, Lazarus GS, Rubin H (1994) Determination of the primary structures of human skin chymase and cathepsin G from cutaneous mast cells of urticaria pigmentosa lesions. J Immunol 152:4062–4069
Baram D, Vaday GG, Salamon P, Drucker I, Hershkoviz R, Mekori YA (2001) Human mast cells release metalloproteinase-9 on contact with activated T cells: juxtacrine regulation by TNF-alpha. J Immunol 167:4008–4016
Silver RB, Reid AC, Mackins CJ, Askwith T, Schaefer U, Herzlinger D, Levi R (2004) Mast cells: a unique source of renin. Proc Natl Acad Sci USA 101:13607–13612
Dell’Italia LJ, Husain A (2002) Dissecting the role of chymase in angiotensin II formation and heart and blood vessel diseases. Curr Opin Cardiol 17:374–379
Li M, Liu K, Michalicek J, Angus JA, Hunt JE, Dell’Italia LJ, Feneley MP, Graham RM, Husain A (2004) Involvement of chymase-mediated angiotensin II generation in blood pressure regulation. J Clin Invest 114:112–120
Pardo J, Wallich R, Ebnet K, Iden S, Zentgraf H, Martin P, Ekiciler A, Prins A, Mullbacher A, Huber M, Simon MM (2007) Granzyme B is expressed in mouse mast cells in vivo and in vitro and causes delayed cell death independent of perforin. Cell Death Differ 14:1768–1779
Gordon JR, Galli SJ (1990) Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature 346:274–276
Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE (1989) Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature 339:64–67
Burd PR, Rogers HW, Gordon JR, Martin CA, Jayaraman S, Wilson SD, Dvorak AM, Galli SJ, Dorf ME (1989) Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med 170:245–257
Echtenacher B, Mannel DN, Hultner L (1996) Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381:75–77
Malaviya R, Ikeda T, Ross E, Abraham SN (1996) Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha [see comments]. Nature 381:77–80
Kunder CA, St John AL, Li G, Leong KW, Berwin B, Staats HF, Abraham SN (2009) Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J Exp Med 206:2455–2467
Piliponsky AM, Chen CC, Grimbaldeston MA, Burns-Guydish SM, Hardy J, Kalesnikoff J, Contag CH, Tsai M, Galli SJ (2010) Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am J Pathol 176:926–938
McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN (2003) Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol 4:1199–1205
Dastych J, Walczak-Drzewiecka A, Wyczolkowska J, Metcalfe DD (1999) Murine mast cells exposed to mercuric chloride release granule-associated N-acetyl-beta-d-hexosaminidase and secrete IL-4 and TNF-alpha. J Allergy Clin Immunol 103:1108–1114
Grutzkau A, Kruger-Krasagakes S, Baumeister H, Schwarz C, Kogel H, Welker P, Lippert U, Henz BM, Moller A (1998) Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell 9:875–884
Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, Dvorak HF, Galli SJ (1998) Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of Fc epsilon receptor I expression. J Exp Med 188:1135–1145
Horsmanheimo L, Harvima IT, Jarvikallio A, Harvima RJ, Naukkarinen A, Horsmanheimo M (1994) Mast cells are one major source of interleukin-4 in atopic dermatitis. Br J Dermatol 131:348–353
Gibbs BF, Arm JP, Gibson K, Lee TH, Pearce FL (1997) Human lung mast cells release small amounts of interleukin-4 and tumour necrosis factor-alpha in response to stimulation by anti-IgE and stem cell factor. Eur J Pharmacol 327:73–78
Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R (1994) Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci USA 91:3739–3743
Orinska Z, Maurer M, Mirghomizadeh F, Bulanova E, Metz M, Nashkevich N, Schiemann F, Schulmistrat J, Budagian V, Giron-Michel J, Brandt E, Paus R, Bulfone-Paus S (2007) IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat Med 13:927–934
Qu Z, Huang X, Ahmadi P, Stenberg P, Liebler JM, Le AC, Planck SR, Rosenbaum JT (1998) Synthesis of basic fibroblast growth factor by murine mast cells. Regulation by transforming growth factor beta, tumor necrosis factor alpha, and stem cell factor. Int Arch Allergy Immunol 115:47–54
Lindstedt KA, Wang Y, Shiota N, Saarinen J, Hyytiainen M, Kokkonen JO, Keski-Oja J, Kovanen PT (2001) Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. Faseb J 15:1377–1388
Zhang S, Anderson DF, Bradding P, Coward WR, Baddeley SM, MacLeod JD, McGill JI, Church MK, Holgate ST, Roche WR (1998) Human mast cells express stem cell factor. J Pathol 186:59–66
Butterfield JH, Weiler D, Peterson EA, Gleich GJ, Leiferman KM (1990) Sequestration of eosinophil major basic protein in human mast cells. Lab Invest 62:77–86
Leiferman KM, Gleich GJ, Kephart GM, Haugen HS, Hisamatsu K, Proud D, Lichtenstein LM, Ackerman SJ (1986) Differences between basophils and mast cells: failure to detect Charcot-Leyden crystal protein (lysophospholipase) and eosinophil granule major basic protein in human mast cells. J Immunol 136:852–855
Christie KN, Stoward PJ (1978) Endogenous peroxidase in mast cells localized with a semipermeable membrane technique. Histochem J 10:425–433
Henderson WR, Kaliner M (1979) Mast cell granule peroxidase: location, secretion, and SRS-A inactivation. J Immunol 122:1322–1328
Rickard A, Lagunoff D (1994) Eosinophil peroxidase accounts for most if not all of the peroxidase activity associated with isolated rat peritoneal mast cells. Int Arch Allergy Immunol 103:365–369
Bashkin P, Razin E, Eldor A, Vlodavsky I (1990) Degranulating mast cells secrete an endoglycosidase that degrades heparan sulfate in subendothelial extracellular matrix. Blood 75:2204–2212
Di Nardo A, Yamasaki K, Dorschner RA, Lai Y, Gallo RL (2008) Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin. J Immunol 180:7565–7573
Braga T, Grujic M, Lukinius A, Hellman L, Abrink M, Pejler G (2007) Serglycin proteoglycan is required for secretory granule integrity in mucosal mast cells. Biochem J 403:49–57
Henningsson F, Hergeth S, Cortelius R, Åbrink M, Pejler G (2006) A role for serglycin proteoglycan in granular retention and processing of mast cell secretory granule components. Febs J 273:4901–4912
Zernichow L, Abrink M, Hallgren J, Grujic M, Pejler G, Kolset SO (2006) Serglycin is the major secreted proteoglycan in macrophages and has a role in the regulation of macrophage tumor necrosis factor-alpha secretion in response to lipopolysaccharide. J Biol Chem 281:26792–26801
Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, Eriksson I, Ledin J, Hellman L, Kjellen L (1999) Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature 400:773–776
Dvorak AM (2005) Piecemeal degranulation of basophils and mast cells is effected by vesicular transport of stored secretory granule contents. Chem Immunol Allergy 85:135–184
Whitaker-Menezes D, Schechter NM, Murphy GF (1995) Serine proteinases are regionally segregated within mast cell granules. Lab Investig 72:34–41
Theoharides TC, Bondy PK, Tsakalos ND, Askenase PW (1982) Differential release of serotonin and histamine from mast cells. Nature 297:229–231
Feyerabend TB, Hausser H, Tietz A, Blum C, Hellman L, Straus AH, Takahashi HK, Morgan ES, Dvorak AM, Fehling HJ, Rodewald HR (2005) Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol Cell Biol 25:6199–6210
Stevens RL, Qui D, McNeil HP, Friend DS, Hunt JE, Austen KF, Zhang J (1996) Transgenic mice that possess a disrupted mast cell protease 5 (mMCP-5) gene can not store carboxypeptidase A (mMC-CPA) protein in their granules. FASEB J 10:17772
Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR (2007) Molecular mechanism of mast cell-mediated innate defense against endothelin and snake venom sarafotoxin. J Exp Med 204:2629–2639
Henningsson F, Wolters P, Chapman HA, Caughey GH, Pejler G (2003) Mast cell cathepsins C and S control levels of carboxypeptidase A and the chymase, mouse mast cell protease 5. Biol Chem 384:1527–1531
Wolters PJ, Pham CT, Muilenburg DJ, Ley TJ, Caughey GH (2001) Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J Biol Chem 276:18551–18556
Rath-Wolfson L (2001) An immunocytochemical approach to the demonstration of intracellular processing of mast cell carboxypeptidase. Appl Immunohistochem Mol Morphol 9:81–85
Zhao W, Oskeritzian CA, Pozez AL, Schwartz LB (2005) Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J Immunol 175:2635–2642
Dvorak AM, Morgan ES, Lichtenstein LM, Weller PF, Schleimer RP (2000) RNA is closely associated with human mast cell secretory granules, suggesting a role(s) for granules in synthetic processes. J Histochem Cytochem 48:1–12
Rundquist I, Allenmark S, Enerback L (1982) Uptake and turnover of dopamine in rat mast cells studied by cytofluorometry and high performance liquid chromatography. Histochem J 14:429–443
Ohtsu H, Kuramasu A, Tanaka S, Terui T, Hirasawa N, Hara M, Makabe-Kobayashi Y, Yamada N, Yanai K, Sakurai E, Okada M, Ohuchi K, Ichikawa A, Nagy A, Watanabe T (2002) Plasma extravasation induced by dietary supplemented histamine in histamine-free mice. Eur J Immunol 32:1698–1708
Arvan P, Castle D (1998) Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J 332(Pt 3):593–610
Prasad P, Yanagihara AA, Small-Howard AL, Turner H, Stokes AJ (2008) Secretogranin III directs secretory vesicle biogenesis in mast cells in a manner dependent upon interaction with chromogranin A. J Immunol 181:5024–5034
Hammel I, Lagunoff D, Galli SJ (2010) Regulation of secretory granule size by the precise generation and fusion of unit granules. J Cell Mol Med (in press)
Grimberg E, Peng Z, Hammel I, Sagi-Eisenberg R (2003) Synaptotagmin III is a critical factor for the formation of the perinuclear endocytic recycling compartment and determination of secretory granules size. J Cell Sci 116:145–154
Schafer T, Starkl P, Allard C, Wolf RM, Schweighoffer T (2010) A granular variant of CD63 is a regulator of repeated human mast cell degranulation. Allergy 65:1242–1255
Hauswirth AW, Escribano L, Prados A, Nunez R, Mirkina I, Kneidinger M, Florian S, Sonneck K, Vales A, Schernthaner GH, Sanchez-Munoz L, Sperr WR, Buhring HJ, Orfao A, Valent P (2008) CD203c is overexpressed on neoplastic mast cells in systemic mastocytosis and is upregulated upon IgE receptor cross-linking. Int J Immunopathol Pharmacol 21:797–806
Dvorak AM (2005) Ultrastructural studies of human basophils and mast cells. J Histochem Cytochem 53:1043–1070
Dvorak AM, Schleimer RP, Lichtenstein LM (1987) Morphologic mast cell cycles. Cell Immunol 105:199–204
Xiang Z, Block M, Lofman C, Nilsson G (2001) IgE-mediated mast cell degranulation and recovery monitored by time-lapse photography. J Allergy Clin Immunol 108:116–121
Tanzola MB, Robbie-Ryan M, Gutekunst CA, Brown MA (2003) Mast cells exert effects outside the central nervous system to influence experimental allergic encephalomyelitis disease course. J Immunol 171:4385–4391
Musch W, Wege AK, Mannel DN, Hehlgans T (2008) Generation and characterization of alpha-chymase-Cre transgenic mice. Genesis 46:163–166
Scholten J, Hartmann K, Gerbaulet A, Krieg T, Muller W, Testa G, Roers A (2008) Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res 17:307–315
Acknowledgments
The authors of this article receive support from The Swedish Research Council, Formas, King Gustaf V:s 80-year Anniversary Fund, Torsten and Ragnar Söderberg Foundation and The Swedish Cancer Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lundequist, A., Pejler, G. Biological implications of preformed mast cell mediators. Cell. Mol. Life Sci. 68, 965–975 (2011). https://doi.org/10.1007/s00018-010-0587-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0587-0