-
PDF
- Split View
-
Views
-
Cite
Cite
David Montani, Laurent Savale, Delphine Natali, Xavier Jaïs, Philippe Herve, Gilles Garcia, Marc Humbert, Gérald Simonneau, Olivier Sitbon, Long-term response to calcium-channel blockers in non-idiopathic pulmonary arterial hypertension, European Heart Journal, Volume 31, Issue 15, August 2010, Pages 1898–1907, https://doi.org/10.1093/eurheartj/ehq170
Close - Share Icon Share
Abstract
To assess the acute vasodilator response and long-term response to calcium-channel blockers (CCB) in pulmonary arterial hypertension (PAH) with associated conditions.
The response to acute vasodilator testing [>20% decrease in mean pulmonary artery pressure (mPAP) and total pulmonary resistance] was assessed in 663 consecutive PAH patients with connective tissue disease (CTD; n = 168), portal hypertension (PoPH; n = 153), anorexigen use (n = 127), human immunodeficiency virus infection (HIV; n = 124), congenital heart disease (CHD; n = 50), and pulmonary veno-occlusive disease or capillary haemangiomatosis (PVOD/PCH; n = 41). An acute vasodilator response was observed in 13.4% of PAH-anorexigen patients, 12.2% of PVOD/PCH, 10.1% of CTD, 1.6% of HIV, 1.3% of PoPH, and was absent in CHD. A long-term response to CCB (marked haemodynamic improvement at 3–4 months and New York Heart Association functional class I or II after 1 year) was reported in 9.4% of PAH-anorexigen patients but was rare in HIV, PoPH, CTD (1.6, 0.7, and 0.6%, respectively) and absent in PVOD/PCH. All patients with a long-term CCB response were alive after 5 years; two deaths not related to PAH occurred after this time. Recent criteria for acute response based on the fall in mPAP to <40 mmHg are more specific to detect long-term responders to CCB.
A long-term CCB response was reported in patients with PAH associated with anorexigen use, but was rare in patients with PoPH or HIV and absent in PVOD/PCH, CHD, and the vast majority of CTD. The prognosis of long-term responders was favourable and related to the underlying cause of PAH.
Introduction
Pulmonary arterial hypertension (PAH) is a disease of the small pulmonary arteries characterized by a progressive increase in pulmonary vascular resistance (PVR) leading to right ventricular failure and death if untreated.1 Although remodelling of the small pulmonary arteries is the main pathological finding in PAH, vasoconstriction plays an important role in the disease pathophysiology, particularly in patients with pulmonary arterial vasoreactivity.1,2 In 1992, Rich et al.3,4 reported that patients with an acute response to calcium-channel blockers (CCB) had dramatically improved survival when treated with CCB long-term compared with patients with no acute response. However, acute vasodilator testing with high-dose CCB can result in serious adverse effects, especially in non-responders. The goal of acute pulmonary vasodilator testing is to identify PAH patients who will respond favourably to long-term CCB therapy. It is now widely accepted that short-acting vasodilators such as inhaled nitric oxide (NO) or intravenous epoprostenol are reliable and safe to test acute pulmonary vasoreactivity.1,5–10
Unfortunately, oral CCB are not always effective in some PAH patients, decreasing cardiac output (CO) and vascular systemic resistance.5 Patients with idiopathic PAH (iPAH) and long-term CCB response have a good outcome, with near-normal survival, justifying acute vasodilator testing. However, <10% of iPAH patients have a long-term response to CCB.5 In a retrospective analysis of 557 iPAH patients tested acutely with intravenous epoprostenol or inhaled NO, only 70 patients (12.6%) displayed acute vasoreactivity, defined as a ≥20% decrease in mean pulmonary artery pressure (mPAP) and PVR, and only 38 of these (6.8% of iPAH patients) had a favourable long-term response to CCB, defined as New York Heart Association (NYHA) functional class I or II with sustained haemodynamic improvement after at least 1 year without the addition of other PAH-specific therapy.5 A decrease in mPAP of ≥10 mmHg to reach an absolute value of <40 mmHg, associated with no change or an increase in CO, is considered the best predictors of long-term response to CCB.5,8–10 Patients with heritable PAH are less likely to exhibit a significant response to acute vasodilator testing.11–13 Although an acute vasodilator response has been described in other forms of PAH,14 recent European Society of Cardiology and European Respiratory Society guidelines concluded that the accuracy of acute vasodilator testing to detect long-term responders to CCB is unclear in patients with other forms of PAH, and no data on long-term response to CCB are available in these patients.8–10 Nevertheless, most experts recommend acute vasodilator testing in all PAH patients.8,9 In other forms of PAH, the proportion of long-term responders to CCB may differ, and, even in the presence of an acute response, initiation of CCB may be deleterious in some associated conditions.15,16 The prevalence of an acute vasodilatory response in these other forms of PAH and the outcome of acute responders treated with CCB are currently largely unknown. The analysis of a large cohort of consecutive PAH patients with associated conditions, in whom acute vasodilator testing was systematically performed and long-term follow-up (at least 5 years) was available, will bring additional information on the clinical relevance of acute testing and the efficacy of CCB long-term therapy in these patients. In addition, we assessed the accuracy of recent criteria used in iPAH to screen long-term responders to CCB in other forms of PAH.
The aims of this study were: (i) to determine the proportion of PAH patients with associated conditions responding during acute vasodilator testing and long-term CCB therapy; (ii) to determine whether an acute response to testing can predict a long-term beneficial effect of CCB in different forms of PAH; (iii) to examine the accuracy of recent criteria used to define acute vasodilator response in iPAH (decrease in mPAP of ≥10 mmHg, reaching an absolute value of <40 mmHg, associated with no change or an increase in CO) in associated forms of PAH5,6; and (iv) to assess the clinical status, haemodynamics, and outcome of long-term CCB responders.
Methods
Subjects
Clinical characteristics, haemodynamic parameters, and response to acute vasodilator testing were reviewed retrospectively in 663 consecutive patients with PAH and associated conditions referred to the French Referral Centre for Pulmonary Hypertension (Université Paris-Sud 11, Hôpital Antoine Béclère, Clamart, France) between June 1984 and May 2003. All patients >15 years of age with pre-capillary pulmonary hypertension confirmed by right-heart catheterization and a diagnosis of PAH associated with anorexigen use, connective tissue disease (CTD), portal hypertension (portopulmonary hypertension, PoPH), human immunodeficiency virus infection (HIV), congenital heart disease (CHD) with systemic-to-pulmonary congenital shunts, and pulmonary veno-occlusive disease (PVOD) or pulmonary capillary haemangiomatosis (PCH) were included. Patients with a diagnosis of iPAH or heritable PAH were excluded from the study. The clinical characteristics at diagnosis and follow-up were stored in the Registry of the French Network of Pulmonary Hypertension. This registry was set up in agreement with French bioethics laws (French Commission Nationale de l'Informatique et des Libertés), and all patients gave their informed consent.17
Baseline evaluation
Pre-capillary pulmonary hypertension was defined as mPAP >25 mmHg with a pulmonary capillary wedge pressure (PCWP) <15 mmHg by right-heart catheterization. Right atrial pressure (RAP), mPAP, PCWP, and mixed venous oxygen saturation (SvO2) were recorded. Cardiac output was measured by the standard thermodilution technique. Total pulmonary resistance (TPR) and PVR were calculated, respectively, as (mPAP)/CO and (mPAP − PCWP)/CO, expressed in Wood Units (WU). Most patients performed a non-encouraged 6-min walk test (6-MWT) according to American Thoracic Society recommendations.
Acute vasodilator testing
Acute vasodilator testing was carried out during right-heart catheterization using inhaled NO (n = 584; 88.1%) or intravenous epoprostenol (n = 79; 11.9%). Because pulmonary artery occlusion pressure could not be recorded consistently during acute vasodilator testing, TPR rather than PVR was used to screen acute responders. At the period of inclusion, an acute vasodilator response was defined as a decrease in both mPAP and resistance of >20% relative to baseline. These criteria were used in these patients to screen acute responders and to initiate CCB. After this period, new criteria defined as a decrease in mPAP of >10 mmHg to reach a level of ≤40 mmHg with normal or increased CO during acute vasodilator testing5,8,9 were proposed to screen acute responders in iPAH. In a second phase of this study, we assessed the accuracy of these recent criteria in patients with non-idiopathic PAH.
Long-term response to calcium-channel blockers
During the period of the study, management of PAH patients followed international recommendations, and treatment with oral CCB was initiated only in patients with acute vasodilator response. Long-term CCB responders were defined as patients who had a marked haemodynamic improvement after 3–4 months of CCB therapy and were in NYHA functional class I or II after at least 1 year on CCB. Initiation of specific PAH therapy was considered in the absence of a long-term response to CCB. In addition, the accuracy of recent criteria based on mPAP and CO to detect long-term CCB responders in other forms of PAH was also investigated. Long-term responders to CCB were followed up for at least 5 years.
Statistical methods
Statistical analysis was performed using Statview 5.0 (SAS Institute, Inc.). Data are presented as mean ± standard deviation (SD) except when stated otherwise. One-way ANOVA was performed for functional and haemodynamic values obtained at baseline, during acute vasodilator testing, at 1 year, and at the last haemodynamic evaluation on CCB monotherapy. P < 0.05 was considered to be statistically significant.
Results
Study population
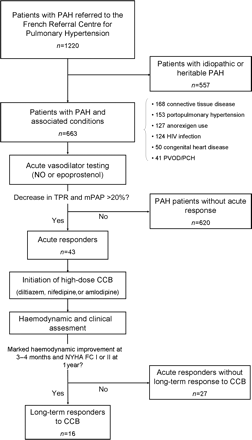
During the study period, 663 consecutive patients with PAH and associated conditions underwent acute vasodilator testing during right-heart catheterization (Figure 1). One hundred and twenty-seven (19.2%) patients had PAH associated with anorexigen use, 153 (23.1%) had PoPH, 124 (18.7%) had HIV infection, 50 (7.5%) had CHD, and 41 (6.2%) had highly probable or confirmed PVOD/PCH. In addition, 168 (25.3%) patients had PAH associated with CTD, including systemic scleroderma (n = 98: 76 limited and 22 diffuse), systemic lupus erythematosus (n = 28), mixed CTD (n = 24), and other CTD (n = 18). The clinical characteristics, including age, sex ratio, NYHA functional class, and 6-MWT results according to PAH-associated condition, are shown in Table 1. Baseline haemodynamic parameters for the different forms of PAH are shown in Table 2.
Clinical characteristics of the study population
| . | Anorexigen (n = 127) . | CTD (n = 168) . | PoPH (n = 153) . | HIV (n = 124) . | CHD (n = 50) . | PVOD/PCH (n = 41) . |
|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 50 ± 12 | 52 ± 16 | 50 ± 11 | 36 ± 7 | 37 ± 12 | 45 ± 18 |
| Gender, f:m | 119:8 | 139:29 | 68:85 | 55:69 | 36:14 | 15:26 |
| NYHA functional class, % | ||||||
| I–II | 16 | 17 | 42 | 38 | 42 | — |
| III | 68 | 59 | 51 | 57 | 58 | 56 |
| IV | 16 | 24 | 7 | 5 | — | 44 |
| 6-MWT, mean ± SD | 248 ± 138 | 260 ± 125 | 314 ± 125 | 357 ± 124 | 343 ± 103 | 222 ± 144 |
| . | Anorexigen (n = 127) . | CTD (n = 168) . | PoPH (n = 153) . | HIV (n = 124) . | CHD (n = 50) . | PVOD/PCH (n = 41) . |
|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 50 ± 12 | 52 ± 16 | 50 ± 11 | 36 ± 7 | 37 ± 12 | 45 ± 18 |
| Gender, f:m | 119:8 | 139:29 | 68:85 | 55:69 | 36:14 | 15:26 |
| NYHA functional class, % | ||||||
| I–II | 16 | 17 | 42 | 38 | 42 | — |
| III | 68 | 59 | 51 | 57 | 58 | 56 |
| IV | 16 | 24 | 7 | 5 | — | 44 |
| 6-MWT, mean ± SD | 248 ± 138 | 260 ± 125 | 314 ± 125 | 357 ± 124 | 343 ± 103 | 222 ± 144 |
CTD, connective tissue disease; PoPH, portopulmonary hypertension; HIV, human immunodeficiency virus; CHD, congenital heart disease; PVOD/PCH, pulmonary veno-occlusive disease or pulmonary capillary haemangiomatosis; NYHA, New York Heart Association; 6-MWT, 6-min walk test.
Clinical characteristics of the study population
| . | Anorexigen (n = 127) . | CTD (n = 168) . | PoPH (n = 153) . | HIV (n = 124) . | CHD (n = 50) . | PVOD/PCH (n = 41) . |
|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 50 ± 12 | 52 ± 16 | 50 ± 11 | 36 ± 7 | 37 ± 12 | 45 ± 18 |
| Gender, f:m | 119:8 | 139:29 | 68:85 | 55:69 | 36:14 | 15:26 |
| NYHA functional class, % | ||||||
| I–II | 16 | 17 | 42 | 38 | 42 | — |
| III | 68 | 59 | 51 | 57 | 58 | 56 |
| IV | 16 | 24 | 7 | 5 | — | 44 |
| 6-MWT, mean ± SD | 248 ± 138 | 260 ± 125 | 314 ± 125 | 357 ± 124 | 343 ± 103 | 222 ± 144 |
| . | Anorexigen (n = 127) . | CTD (n = 168) . | PoPH (n = 153) . | HIV (n = 124) . | CHD (n = 50) . | PVOD/PCH (n = 41) . |
|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 50 ± 12 | 52 ± 16 | 50 ± 11 | 36 ± 7 | 37 ± 12 | 45 ± 18 |
| Gender, f:m | 119:8 | 139:29 | 68:85 | 55:69 | 36:14 | 15:26 |
| NYHA functional class, % | ||||||
| I–II | 16 | 17 | 42 | 38 | 42 | — |
| III | 68 | 59 | 51 | 57 | 58 | 56 |
| IV | 16 | 24 | 7 | 5 | — | 44 |
| 6-MWT, mean ± SD | 248 ± 138 | 260 ± 125 | 314 ± 125 | 357 ± 124 | 343 ± 103 | 222 ± 144 |
CTD, connective tissue disease; PoPH, portopulmonary hypertension; HIV, human immunodeficiency virus; CHD, congenital heart disease; PVOD/PCH, pulmonary veno-occlusive disease or pulmonary capillary haemangiomatosis; NYHA, New York Heart Association; 6-MWT, 6-min walk test.
Haemodynamic parameters at baseline
| . | Anorexigen (n = 127) . | CTD (n = 168) . | PoPH (n = 153) . | HIV (n = 124) . | CHD (n = 50) . | PVOD/PCH (n = 41) . |
|---|---|---|---|---|---|---|
| Baseline haemodynamic | ||||||
| RAP (mmHg) | 11 ± 6 | 8 ± 5 | 10 ± 6 | 9 ± 5 | 7 ± 5 | 9 ± 5 |
| mPAP (mmHg) | 61 ± 12 | 50 ± 10 | 53 ± 12 | 51 ± 11 | 69 ± 19 | 59 ± 11 |
| PCWP (mmHg) | 9 ± 3 | 7 ± 3 | 9 ± 3 | 7 ± 3 | 7 ± 5 | 7 ± 3 |
| CO (L/min/m2) | 2.2 ± 0.6 | 2.4 ± 0.7 | 3.0 ± 0.9 | 2.7 ± 0.7 | 2.5 ± 0.9 | 2.2 ± 0.6 |
| TPR (Wood Unit) | 17.2 ± 6.8 | 14.6 ± 6.4 | 11.0 ± 4.8 | 12.4 ± 5.1 | 21.6 ± 13 | 18.1 ± 8.1 |
| SvO2 (%) | 59 ± 10 | 60 ± 11 | 65 ± 9 | 59 ± 10 | 65 ± 10 | 57 ± 9 |
| Acute vasodilator testing | ||||||
| Decrease in mPAP and TPR >20%a [n (%)] | 17 (13.4) | 17 (10.1) | 2 (1.3) | 2 (1.6) | 0 | 5 (12.2) |
| Long-term response to CCBb (n) | 12 | 1 | 1 | 2 | 0 | 0 |
| Proportion (%) of long-term responders among acute responders | 70.6 | 5.8 | 50 | 100 | 0 | 0 |
| Proportion (%) of long-term responders among overall population | 9.4 | 0.6 | 0.7 | 1.6 | 0 | 0 |
| . | Anorexigen (n = 127) . | CTD (n = 168) . | PoPH (n = 153) . | HIV (n = 124) . | CHD (n = 50) . | PVOD/PCH (n = 41) . |
|---|---|---|---|---|---|---|
| Baseline haemodynamic | ||||||
| RAP (mmHg) | 11 ± 6 | 8 ± 5 | 10 ± 6 | 9 ± 5 | 7 ± 5 | 9 ± 5 |
| mPAP (mmHg) | 61 ± 12 | 50 ± 10 | 53 ± 12 | 51 ± 11 | 69 ± 19 | 59 ± 11 |
| PCWP (mmHg) | 9 ± 3 | 7 ± 3 | 9 ± 3 | 7 ± 3 | 7 ± 5 | 7 ± 3 |
| CO (L/min/m2) | 2.2 ± 0.6 | 2.4 ± 0.7 | 3.0 ± 0.9 | 2.7 ± 0.7 | 2.5 ± 0.9 | 2.2 ± 0.6 |
| TPR (Wood Unit) | 17.2 ± 6.8 | 14.6 ± 6.4 | 11.0 ± 4.8 | 12.4 ± 5.1 | 21.6 ± 13 | 18.1 ± 8.1 |
| SvO2 (%) | 59 ± 10 | 60 ± 11 | 65 ± 9 | 59 ± 10 | 65 ± 10 | 57 ± 9 |
| Acute vasodilator testing | ||||||
| Decrease in mPAP and TPR >20%a [n (%)] | 17 (13.4) | 17 (10.1) | 2 (1.3) | 2 (1.6) | 0 | 5 (12.2) |
| Long-term response to CCBb (n) | 12 | 1 | 1 | 2 | 0 | 0 |
| Proportion (%) of long-term responders among acute responders | 70.6 | 5.8 | 50 | 100 | 0 | 0 |
| Proportion (%) of long-term responders among overall population | 9.4 | 0.6 | 0.7 | 1.6 | 0 | 0 |
CTD, connective tissue disease; PoPH, portopulmonary hypertension; HIV, human immunodeficiency virus; CHD, congenital heart disease; PVOD/PCH, pulmonary veno-occlusive disease or pulmonary capillary haemangiomatosis; RAP, right atrial pressure; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; CO, cardiac output; TPR, total pulmonary resistance; SvO2, mixed venous oxygen saturation; CCB, calcium-channel blockers.
Results are expressed as mean ± SD unless stated otherwise.
aAn acute vasodilator response was defined as a decrease in both mPAP and TPR of >20% relative to baseline values.
bLong-term responders to CCB were defined as patients who had a marked haemodynamic improvement after 3–4 months on CCB and were in NYHA functional class I or II after at least 1 year.
Haemodynamic parameters at baseline
| . | Anorexigen (n = 127) . | CTD (n = 168) . | PoPH (n = 153) . | HIV (n = 124) . | CHD (n = 50) . | PVOD/PCH (n = 41) . |
|---|---|---|---|---|---|---|
| Baseline haemodynamic | ||||||
| RAP (mmHg) | 11 ± 6 | 8 ± 5 | 10 ± 6 | 9 ± 5 | 7 ± 5 | 9 ± 5 |
| mPAP (mmHg) | 61 ± 12 | 50 ± 10 | 53 ± 12 | 51 ± 11 | 69 ± 19 | 59 ± 11 |
| PCWP (mmHg) | 9 ± 3 | 7 ± 3 | 9 ± 3 | 7 ± 3 | 7 ± 5 | 7 ± 3 |
| CO (L/min/m2) | 2.2 ± 0.6 | 2.4 ± 0.7 | 3.0 ± 0.9 | 2.7 ± 0.7 | 2.5 ± 0.9 | 2.2 ± 0.6 |
| TPR (Wood Unit) | 17.2 ± 6.8 | 14.6 ± 6.4 | 11.0 ± 4.8 | 12.4 ± 5.1 | 21.6 ± 13 | 18.1 ± 8.1 |
| SvO2 (%) | 59 ± 10 | 60 ± 11 | 65 ± 9 | 59 ± 10 | 65 ± 10 | 57 ± 9 |
| Acute vasodilator testing | ||||||
| Decrease in mPAP and TPR >20%a [n (%)] | 17 (13.4) | 17 (10.1) | 2 (1.3) | 2 (1.6) | 0 | 5 (12.2) |
| Long-term response to CCBb (n) | 12 | 1 | 1 | 2 | 0 | 0 |
| Proportion (%) of long-term responders among acute responders | 70.6 | 5.8 | 50 | 100 | 0 | 0 |
| Proportion (%) of long-term responders among overall population | 9.4 | 0.6 | 0.7 | 1.6 | 0 | 0 |
| . | Anorexigen (n = 127) . | CTD (n = 168) . | PoPH (n = 153) . | HIV (n = 124) . | CHD (n = 50) . | PVOD/PCH (n = 41) . |
|---|---|---|---|---|---|---|
| Baseline haemodynamic | ||||||
| RAP (mmHg) | 11 ± 6 | 8 ± 5 | 10 ± 6 | 9 ± 5 | 7 ± 5 | 9 ± 5 |
| mPAP (mmHg) | 61 ± 12 | 50 ± 10 | 53 ± 12 | 51 ± 11 | 69 ± 19 | 59 ± 11 |
| PCWP (mmHg) | 9 ± 3 | 7 ± 3 | 9 ± 3 | 7 ± 3 | 7 ± 5 | 7 ± 3 |
| CO (L/min/m2) | 2.2 ± 0.6 | 2.4 ± 0.7 | 3.0 ± 0.9 | 2.7 ± 0.7 | 2.5 ± 0.9 | 2.2 ± 0.6 |
| TPR (Wood Unit) | 17.2 ± 6.8 | 14.6 ± 6.4 | 11.0 ± 4.8 | 12.4 ± 5.1 | 21.6 ± 13 | 18.1 ± 8.1 |
| SvO2 (%) | 59 ± 10 | 60 ± 11 | 65 ± 9 | 59 ± 10 | 65 ± 10 | 57 ± 9 |
| Acute vasodilator testing | ||||||
| Decrease in mPAP and TPR >20%a [n (%)] | 17 (13.4) | 17 (10.1) | 2 (1.3) | 2 (1.6) | 0 | 5 (12.2) |
| Long-term response to CCBb (n) | 12 | 1 | 1 | 2 | 0 | 0 |
| Proportion (%) of long-term responders among acute responders | 70.6 | 5.8 | 50 | 100 | 0 | 0 |
| Proportion (%) of long-term responders among overall population | 9.4 | 0.6 | 0.7 | 1.6 | 0 | 0 |
CTD, connective tissue disease; PoPH, portopulmonary hypertension; HIV, human immunodeficiency virus; CHD, congenital heart disease; PVOD/PCH, pulmonary veno-occlusive disease or pulmonary capillary haemangiomatosis; RAP, right atrial pressure; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; CO, cardiac output; TPR, total pulmonary resistance; SvO2, mixed venous oxygen saturation; CCB, calcium-channel blockers.
Results are expressed as mean ± SD unless stated otherwise.
aAn acute vasodilator response was defined as a decrease in both mPAP and TPR of >20% relative to baseline values.
bLong-term responders to CCB were defined as patients who had a marked haemodynamic improvement after 3–4 months on CCB and were in NYHA functional class I or II after at least 1 year.
Flow chart of patients with pulmonary arterial hypertension (PAH) and associated conditions referred to the French Referral Centre for Pulmonary hypertension.
Acute vasodilator testing
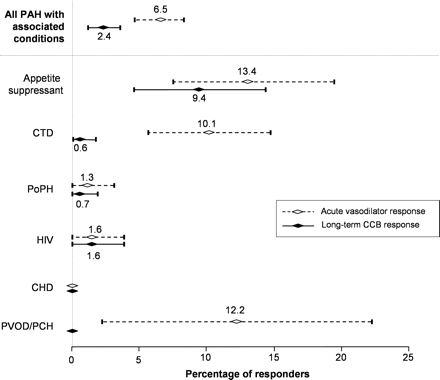
Of the 663 consecutive patients who underwent acute vasodilator testing, 43 [6.5% (95% CI 4.6–8.4)] had an acute response, defined by a decrease in both mPAP and TPR of ≥20% compared with baseline values. The proportion of acute responders was dependent on the PAH-associated condition (Figure 2). An acute response was frequently observed in PAH associated with anorexigen use [n = 17; 13.4% (95% CI 7.5–19.3)] and PVOD/PCH [n = 5; 12.2% (95% CI 2.2–22.2)], but was rarely observed in patients with CTD [n = 17, 10.1% (95% CI 5.6–14.7), including 11 limited systemic sclerosis, 1 diffuse systemic sclerosis, 2 mixed CTD, 2 Sjögren syndrome, 1 rheumatoid arthritis], PoPH [n = 2; 1.3% (95% CI 0–3.1)], or HIV infection [n = 2; 1.6% (95% CI 0–3.8)]. No acute response was reported in patients with PAH associated with CHD (Figure 2).
Percentage of acute vasodilator responders and long-term responders to CCB in PAH patients according to their underlying conditions. Results (mean and 95% CI) are expressed as percentage of patients with acute vasodilator response (dashed lines) and long-term response to CCB (solid lines) in each condition associated with PAH. Acute vasodilator responders are defined as patients showing a decrease in both mPAP and total pulmonary resistance (TPR) of >20% relative to baseline. Long-term responders to CCB are defined as patients with sustained haemodynamic improvement after 3 months on CCB and with NYHA functional class I or II after at least 1 year on CCB monotherapy. CCB, calcium channel blockers; CHD, congenital heart disease; CTD, connective tissue diseases; HIV, human immunodeficiency virus; mPAP, mean pulmonary artery pressure; PAH, pulmonary arterial hypertension; PCH, pulmonary capillary haemangiomatosis; PoPH, portopulmonary hypertension; PVOD, pulmonary veno-occlusive disease.
Long-term calcium-channel blocker responders
In the overall population, 16 PAH patients [2.4% (95% CI 1.2–3.6)] were considered long-term CCB responders: 12 [9.4% (95% CI 4.4–14.5)] with anorexigen use, 2 [1.6% (95% CI 0–3.8)] with HIV infection, 1 [0.7% (95% CI 0–1.9)] with PoPH, and 1 [0.6% (95% CI 0–1.8)] with CTD (Sjögren syndrome). At the time of initiation of CCB, the 16 long-term responders received diltiazem (n = 9), nifedipine (n = 6), and amlodipine (n = 1). After haemodynamic stabilization, most patients received diltiazem (n = 13) with a median dose of 480 mg/day (range 240–720 mg/day), two patients received nifedipine (dose of 60 and 90 mg/day, respectively), and one amlodipine (dose of 20 mg/day). Although an acute response was frequently observed in PVOD/PCH, no PVOD or PCH patient had a long-term response to CCB. Indeed, all five PVOD/PCH acute responders experienced a rapid clinical deterioration with CCB and three developed severe pulmonary oedema requiring intensive care. In PAH associated with CTD, two patients had a histologically confirmed diagnosis of PVOD and one had a highly probable PVOD. For the majority of CTD patients, CCB were stopped in the first 4 months following initiation because of clinical or haemodynamic deterioration. One CTD patient with highly probable diagnosis of PVOD experienced severe pulmonary oedema in the first week following the initiation of CCB, requiring management in the intensive care unit with vasoactive drugs and high-dose diuretics. Because of the absence of an acute response in PAH patients with CHD, none of these patients received CCB.
In PAH associated with anorexigen use, 70.6% of patients with an acute vasodilator response (defined as a decrease in mPAP and TPR >20% relative to baseline) benefited from long-term CCB therapy (Figure 2). In other forms of PAH, the rate of long-term responders to CCB was extremely low (Figure 2).
Comparison of criteria for defining acute vasodilator response
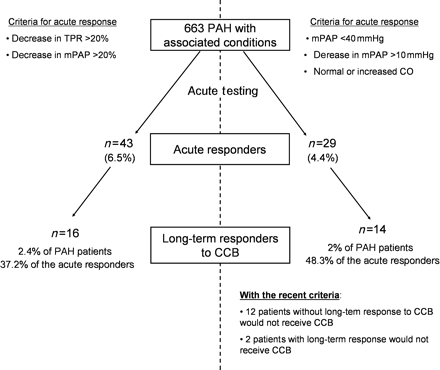
With an acute vasodilator response defined by a decrease in both mPAP and TPR ≥20%, 43 patients had an acute response. Using the recent criteria (decrease in mPAP >10 mmHg to reach a value of <40 mmHg and a normal or increased CO), 29 patients (4.4% of the overall population) would be considered acute responders. Among these, 14 met the criteria for long-term response to CCB (Figure 3). Using these criteria, two patients (one PAH patient with anorexigen use and one PoPH patient) who had long-term response to CCB in our cohort would not have been considered acute responders and would not have received CCB. Both of these two patients had an acute response to inhaled NO as follows: 27% decrease in mPAP to 47 mmHg, and 21% decrease to 46 mmHg, respectively. Interestingly, although these two patients showed clinical improvement after 1 year on CCB, both experienced clinical and haemodynamic deterioration requiring additional treatment with continuous intravenous epoprostenol after 22 months in one case and sildenafil and inhaled iloprost after 58 months in the other.
Comparison of criteria for defining acute vasodilator response. Proportions of acute vasodilator responders and long-term responders to calcium-channel blockers (CCB) screened with criteria based on a decrease in mean pulmonary artery pressure (mPAP) and total pulmonary resistance (TPR) ≥20% are presented in the left panel. Using the criteria defined by a decrease in mPAP >10 mmHg to reach a value of <40 mmHg and a normal or increased CO (right panel), 29 patients would be considered acute responders and 14 of them met the criteria for long-term response to calcium-channel blockers. The specificity of these criteria to detect long-term responders in pulmonary arterial hypertension (PAH) with associated conditions was 37.2 and 48.3%, respectively. Using the recent criteria, the initiation of potentially detrimental calcium-channel blocker therapy would be avoided in 13 pulmonary arterial hypertension patients with a confirmed absence of long-term calcium-channel blocker response, and two patients with long-term response would not receive calcium-channel blockers.
Thus, the criteria used in iPAH, based on an absolute value of mPAP and CO, were more specific to detect long-term responders compared with a >20% decrease in mPAP and TPR (specificity of 48.3 vs. 37.2%, respectively) in patients displaying PAH with associated conditions. The specificity was higher in PAH associated with anorexigen use, where 13 patients fulfilled these criteria and 11 of them had a long-term response to CCB (specificity of 84.6% compared with 70.6% for criteria based on mPAP and TPR). Although the specificity of recent criteria is low in the overall population of PAH patients with associated conditions, the use of these criteria would have avoided the initiation of potentially detrimental CCB therapy in 12 PAH patients with a confirmed absence of long-term CCB response (Figure 3).
Evolution of clinical, functional, and haemodynamic parameters in long-term calcium-channel blocker responders
The evolution of clinical, functional, and haemodynamic parameters in long-term CCB responders is shown in Table 3. Haemodynamic parameters including mPAP, CO, TPR, PVR, and SvO2 were significantly improved after acute testing, after 1 year, and at the last haemodynamic evaluation on CCB monotherapy (75 ± 47 months after the initiation). The 6-MWT was significantly improved after 1 year and at the last evaluation.
Clinical, functional, and haemodynamic effects of acute vasodilator testing and long-term calcium-channel blocker monotherapy in long-term responders (n = 16)
| . | Initial evaluation . | 1 Year evaluation . | Last haemodynamic evaluation on CCB monotherapya . | |
|---|---|---|---|---|
| . | Baseline . | Acute testing . | ||
| mPAP (mmHg) | 51 ± 8 | 32 ± 9* | 37 ± 8* | 38 ± 16** |
| CO (L/m2) | 2.8 ± 0.7 | 3.0 ± 0.7*** | 3.3 ± 0.6*** | 3.5 ± 0.7** |
| SI (mL/m2) | 37 ± 11 | 43 ± 12* | 48 ± 10* | 50 ± 13** |
| TPR (Wood Unit) | 11.2 ± 3.6 | 6.4 ± 2.5* | 6.7 ± 2.7* | 6.9 ± 4.9** |
| PVR (Wood Unit) | 9.5 ± 3.0 | 4.8 ± 2.0* | 5.2 ± 2.5* | 5.7 ± 4.9** |
| SvO2 (%) | 66 ± 9 | 70 ± 5** | 71 ± 7*** | 70 ± 7 |
| 6-MWT | ||||
| Distance (m) | 315 ± 130 | 415 ± 91** | 431 ± 74** | |
| Nadir SpO2 (%) | 92 (79.5–95.5) | 92 (89.3–94.5) | 93 (91–96) | |
| NYHA functional class (n) | ||||
| I | — | 2 | 5 | |
| II | 3 | 14 | 9 | |
| III | 11 | — | 2 | |
| IV | 2 | — | — | |
| . | Initial evaluation . | 1 Year evaluation . | Last haemodynamic evaluation on CCB monotherapya . | |
|---|---|---|---|---|
| . | Baseline . | Acute testing . | ||
| mPAP (mmHg) | 51 ± 8 | 32 ± 9* | 37 ± 8* | 38 ± 16** |
| CO (L/m2) | 2.8 ± 0.7 | 3.0 ± 0.7*** | 3.3 ± 0.6*** | 3.5 ± 0.7** |
| SI (mL/m2) | 37 ± 11 | 43 ± 12* | 48 ± 10* | 50 ± 13** |
| TPR (Wood Unit) | 11.2 ± 3.6 | 6.4 ± 2.5* | 6.7 ± 2.7* | 6.9 ± 4.9** |
| PVR (Wood Unit) | 9.5 ± 3.0 | 4.8 ± 2.0* | 5.2 ± 2.5* | 5.7 ± 4.9** |
| SvO2 (%) | 66 ± 9 | 70 ± 5** | 71 ± 7*** | 70 ± 7 |
| 6-MWT | ||||
| Distance (m) | 315 ± 130 | 415 ± 91** | 431 ± 74** | |
| Nadir SpO2 (%) | 92 (79.5–95.5) | 92 (89.3–94.5) | 93 (91–96) | |
| NYHA functional class (n) | ||||
| I | — | 2 | 5 | |
| II | 3 | 14 | 9 | |
| III | 11 | — | 2 | |
| IV | 2 | — | — | |
CCB, calcium-channel blocker; mPAP, mean pulmonary artery pressure; CO, cardiac output; SI, systolic index; TPR, total pulmonary resistance; PVR, pulmonary vascular resistance; SvO2, mixed venous oxygen saturation; 6-MWT, 6-min walk test; Nadir SpO2, lowest pulse oxygen saturation during 6-MWT; NYHA, New York Heart Association.
All values shown are mean ± SD, except nadir SpO2 expressed as median (inter-quartile range).
aAt 75 ± 47 months.
*P < 0.001 compared with baseline; **P < 0.01 compared with baseline; ***P < 0.05 compared with baseline.
Clinical, functional, and haemodynamic effects of acute vasodilator testing and long-term calcium-channel blocker monotherapy in long-term responders (n = 16)
| . | Initial evaluation . | 1 Year evaluation . | Last haemodynamic evaluation on CCB monotherapya . | |
|---|---|---|---|---|
| . | Baseline . | Acute testing . | ||
| mPAP (mmHg) | 51 ± 8 | 32 ± 9* | 37 ± 8* | 38 ± 16** |
| CO (L/m2) | 2.8 ± 0.7 | 3.0 ± 0.7*** | 3.3 ± 0.6*** | 3.5 ± 0.7** |
| SI (mL/m2) | 37 ± 11 | 43 ± 12* | 48 ± 10* | 50 ± 13** |
| TPR (Wood Unit) | 11.2 ± 3.6 | 6.4 ± 2.5* | 6.7 ± 2.7* | 6.9 ± 4.9** |
| PVR (Wood Unit) | 9.5 ± 3.0 | 4.8 ± 2.0* | 5.2 ± 2.5* | 5.7 ± 4.9** |
| SvO2 (%) | 66 ± 9 | 70 ± 5** | 71 ± 7*** | 70 ± 7 |
| 6-MWT | ||||
| Distance (m) | 315 ± 130 | 415 ± 91** | 431 ± 74** | |
| Nadir SpO2 (%) | 92 (79.5–95.5) | 92 (89.3–94.5) | 93 (91–96) | |
| NYHA functional class (n) | ||||
| I | — | 2 | 5 | |
| II | 3 | 14 | 9 | |
| III | 11 | — | 2 | |
| IV | 2 | — | — | |
| . | Initial evaluation . | 1 Year evaluation . | Last haemodynamic evaluation on CCB monotherapya . | |
|---|---|---|---|---|
| . | Baseline . | Acute testing . | ||
| mPAP (mmHg) | 51 ± 8 | 32 ± 9* | 37 ± 8* | 38 ± 16** |
| CO (L/m2) | 2.8 ± 0.7 | 3.0 ± 0.7*** | 3.3 ± 0.6*** | 3.5 ± 0.7** |
| SI (mL/m2) | 37 ± 11 | 43 ± 12* | 48 ± 10* | 50 ± 13** |
| TPR (Wood Unit) | 11.2 ± 3.6 | 6.4 ± 2.5* | 6.7 ± 2.7* | 6.9 ± 4.9** |
| PVR (Wood Unit) | 9.5 ± 3.0 | 4.8 ± 2.0* | 5.2 ± 2.5* | 5.7 ± 4.9** |
| SvO2 (%) | 66 ± 9 | 70 ± 5** | 71 ± 7*** | 70 ± 7 |
| 6-MWT | ||||
| Distance (m) | 315 ± 130 | 415 ± 91** | 431 ± 74** | |
| Nadir SpO2 (%) | 92 (79.5–95.5) | 92 (89.3–94.5) | 93 (91–96) | |
| NYHA functional class (n) | ||||
| I | — | 2 | 5 | |
| II | 3 | 14 | 9 | |
| III | 11 | — | 2 | |
| IV | 2 | — | — | |
CCB, calcium-channel blocker; mPAP, mean pulmonary artery pressure; CO, cardiac output; SI, systolic index; TPR, total pulmonary resistance; PVR, pulmonary vascular resistance; SvO2, mixed venous oxygen saturation; 6-MWT, 6-min walk test; Nadir SpO2, lowest pulse oxygen saturation during 6-MWT; NYHA, New York Heart Association.
All values shown are mean ± SD, except nadir SpO2 expressed as median (inter-quartile range).
aAt 75 ± 47 months.
*P < 0.001 compared with baseline; **P < 0.01 compared with baseline; ***P < 0.05 compared with baseline.
Outcome of long-term responders
In the 16 patients who met the criteria for long-term response to CCB after 1 year, 5-year survival was 100%. During follow-up (median 154 months, range 63–289), two patients had died but their deaths were not related to PAH: one patient with PoPH died from advanced hepatocellular carcinoma, and one with Sjögren syndrome died from metastatic rectal cancer, after 63 and 68 months, respectively. During follow-up, three patients experienced clinical deterioration requiring treatment escalation. One patient with PoPH was started on continuous intravenous epoprostenol 22 months after the initiation of CCB, and two patients with anorexigen use were started on sildenafil and iloprost after 59 months in one case, and bosentan followed by inhaled treprostinil after 74 months in the other.
There were 12 long-term responders to CCB among patients with PAH associated with anorexigen use; all patients were alive, and 10 remained on CCB monotherapy after a median time of 169 months (range 81–289 months). Two PAH patients with HIV infection remained on CCB monotherapy after 99 and 107 months, respectively.
Discussion
To our knowledge, this study is the first report of a large cohort of PAH patients with associated conditions in whom acute testing was performed and long-term response to CCB (NYHA functional status, exercise capacity, haemodynamics) and the 5-year survival available. In this cohort, we demonstrated that even if an acute vasodilator response is frequent in PAH with associated conditions, long-term response to CCB is extremely rare and depends on the underlying condition. In some conditions such as CHD, PVOD/PCH, or the majority of CTD, an acute response is not predictive of long-term response, and acute testing might not be useful. However, some patients have a sustained response to CCB with prolonged survival, as observed in iPAH, particularly in PAH associated with anorexigen use, and also in PoPH and PAH associated with HIV infection. This study brought additional information useful in the management of PAH patients in confirming that CCB is a safe and efficacious therapy in some PAH patients with associated conditions. In contrast, CCB is less useful in other subgroups of patients (CTD, CHD, and PVOD/PCH) and may result in serious adverse effects in PVOD/PCH. In a previous report,5 we have shown that criteria based on CO and an absolute value of mPAP are more specific to detect long-term responders to CCB, but these criteria were evaluated only in iPAH, with no data available in other forms of PAH. In the present study, we therefore confirmed that these criteria are more accurate to detect long-term responders to CCB in PAH with associated conditions.
The rate of responders to acute vasodilator testing and long-term CCB among PAH patients using anorexigen was similar to that described previously in iPAH.5 This study also demonstrated that the acute vasodilator response in PAH associated with PVOD or CTD is similar to that in iPAH, but that a long-term response to CCB is rare in CTD and absent in PVOD. As reported previously,15,16,18 initiation of CCB therapy in PVOD patients with an acute vasodilator response always led to clinical deterioration with the likelihood of severe pulmonary oedema. This study also confirms that the recent criteria based on mPAP and CO, proposed for iPAH,5 are more specific than a >20% decrease in mPAP and TPR to detect long-term CCB responders in PAH with associated conditions.
The proportion of acute vasodilator responders and long-term CCB responders among PAH patients with associated conditions has not been evaluated previously. Indeed, our study provides data on long-term CCB response in PAH associated with anorexigen use, which represents 9.5% of the overall population in the French National Registry.17,19 Souza et al.20 recently demonstrated that PAH associated with anorexigen shares clinical, functional, haemodynamic, and genetic features with iPAH, suggesting that fenfluramine exposure represents a potential trigger for PAH without influencing its clinical course. As suggested previously,20 this study confirms that an acute response to vasodilator testing may occur in PAH associated with anorexigen use and that the rate of acute responders is similar to that reported in iPAH (11.8 and 12.6%, respectively).5
No acute vasodilator response was observed in the 41 adult patients with CHD in this study. It has previously been suggested that an acute vasodilator response may be more frequent in the early stages of the disease,21 and this could explain why adults with a long history of PAH associated with CHD had no acute vasodilator response. Another study including eight children with iPAH and CHD also suggested that an acute response is rare.22 The difference between the rates of acute response in iPAH and PAH associated with CHD may be partly due to the differences in pathophysiology between these two entities. In addition, the small decrease in mPAP sometimes observed with inhaled NO in these usually hypoxemic patients may be due to the relief of hypoxic vasoconstriction and may be misinterpreted as acute vasoreactivity indicating CCB therapy.
Interestingly, PVOD and PCH were associated with a similar proportion (12%) of acute vasodilator responders as iPAH. However, no PVOD or PCH patients had a long-term CCB response and all patients deteriorated after the initiation of CCB monotherapy. These results are consistent with previous reports demonstrating that an acute vasodilator response may be observed in PVOD patients and that a degree of vasoreactivity is frequently observed.15,16,23 In three PVOD/PCH patients, severe pulmonary oedema occurred in the first days following the initiation of CCB, as described previously with specific PAH therapies.15,16,24 This suggests that an acute vasodilator response in PVOD or PCH may not be predictive of a good prognosis and that CCB should not be used in PVOD, even in the context of a positive acute test. In a series of 24 histologically confirmed PVOD patients, it was recently demonstrated that the acute vasodilator test was unable to predict those who later developed pulmonary oedema following the initiation of PAH-specific therapy.15 Therefore, it can be argued that vasoreactivity testing has no role in the investigation of PVOD patients and will not predict those at risk of developing pulmonary oedema. It is crucial to identify this subset of PAH patients because of the risk associated with the initiation of CCB. Recent reports have suggested that high-resolution computed tomography of the chest, arterial blood gases, pulmonary function tests, and bronchoalveolar lavage could be useful to identify PAH patients with a high probability of PVOD.15,16 CCB remain contraindicated in PVOD or PCH, even if an acute vasodilator response is observed.
In this study, the proportion of CTD patients with an acute vasodilator response was similar to that in iPAH. However, 90% of our patients with CTD had systemic scleroderma, systemic lupus erythematosus, or mixed CTD, and none had a long-term response to CCB. Only one patient with Sjögren syndrome benefited from long-term CCB therapy. A possible short-term response to CCB has been reported in PAH patients with Sjögren syndrome; however, data on long-term response are scarce.25 Our patient with PAH and Sjögren syndrome experienced a sustained haemodynamic improvement; however, the long-term effect of CCB was not easy to determine because the patient was also receiving concomitant immunosuppressive therapy. It has been demonstrated that occlusive venopathy may occur in severe PAH associated with different conditions and is relatively frequent in PAH associated with CTD such as systemic sclerosis.26,27 Interestingly, three of our CTD patients with an acute vasodilator response had a confirmed or highly probable diagnosis of PVOD and one of them experienced severe pulmonary oedema in the first week following the initiation of CCB. In addition, most of these CTD patients experienced a rapid clinical and haemodynamic deterioration in the first months following the initiation of CCB. The absence of long-term response in CTD-associated PAH may be at least in part related to the frequent venous or capillary involvement observed in these conditions. Although the proportion of long-term responders to CCB was low in patients with HIV infection or PoPH, acute vasodilator testing is important in the management of these patients because of its ability to detect long-term response to oral high-dose CCB. In this study, all patients who experienced a long-term response to CCB had a better prognosis and were alive after 5 years, demonstrating a similar prognosis to that reported in iPAH patients with a long-term response to CCB.5
Three different situations may therefore be considered. First, for PAH associated with anorexigen use, the same proportion of long-term responders to CCB was observed as in iPAH. Acute vasodilator testing should be performed in these patients as is recommended for iPAH in the European Guidelines.8,9 Second, in PVOD/PCH, and in PAH associated with CHD or CTD, no long-term responders to CCB were observed (except in a rare form of PAH associated with Sjögren syndrome). In addition, all PVOD/PCH patients and some patients with PAH associated with CTD may experience severe pulmonary oedema or clinical deterioration with CCB therapy. In this context, acute vasodilator testing probably plays no role in the clinical management of patients with PVOD/PCH, PAH associated with CHD, or CTD. Third, even though it was uncommon (∼1.5%), we confirmed that PoPH and HIV-associated PAH may be associated with long-term response to CCB.
These patients with long-term response to CCB were alive after 5 years, and their prognosis seems not related to PAH but to the prognosis of the underlying conditions. The retrospective design of the study does not allow comparing survival of responders and non-responders. However, we have previously demonstrated in iPAH patients that long-term response to CCB is strongly associated with a better prognosis (5-year survival >90% in long-term responders vs. 48% in the absence of long-term response). Indeed, we reported that 1-, 2-, and 3-year survival in the French Registry were 82.9%, 67.1%, and 58.2%, respectively.28 Furthermore, the prognosis of PAH with associated conditions is usually considered to be worse than the prognosis of iPAH. In this study, we observed a 100% 5-year survival in the 16 long-term responders to CCB greater than survival expected in this population, suggesting that long-term response to CCB may also be a factor of better prognosis in PAH with associated conditions. Indeed, potential effects of PAH-specific therapies (ERAs, PDE5 inhibitors, prostacyclin derivatives) have never been demonstrated in acute vasodilator responders. Even if bosentan has been demonstrated to be efficacious in patients with HIV-associated PAH, no correlation has ever been found between the magnitude of acute vasodilator response and the long-term response to bosentan. Currently, there are no data suggesting that patients who respond long-term to CCB are able to respond to PAH-specific therapies. In our experience, we observed few patients who were not initially tested acutely with NO and who failed on PAH-specific therapy. In those patients, the demonstration of an acute vasodilator response leading to the initiation of CCB was followed by a dramatic improvement on that drug. Thus, to not detect these long-term responders to CCB might affect their prognosis, and acute vasodilator testing should be performed according to the local availability.
In this study, all long-term responders received high-dose CCB, and the vast majority received diltiazem in the course of the disease (median dose 480 mg/day, n = 13); the three other patients received nifedipine (n = 2; dose of 60 and 90 mg/day, respectively) or amlodipine (dose of 20 mg/day). Despite the long period of this retrospective study, our management of CCB in this study is still in accordance with recent ERS/ESC recommendations for the management of iPAH patients with acute vasodilator response.8,9 These recommendations reported that the CCB predominantly used in the literature are nifedipine, diltiazem, and amlodipine, with particular emphasis on the first two and that the choice of CCB is based upon the patient's heart rate at baseline (a relative bradycardia favouring nifedipine and amlodipine and a relative tachycardia favouring diltiazem).8,9 Today, we used high-dose of the same ‘old’ CCBs (diltiazem, nifedipine, and amlodipine) for the rare PAH patients with acute vasodilator response. We have no experience of the use of the more recent CCB in this context.
This retrospective study included patients displaying PAH with associated conditions before 2003. In this period, all PAH patients referred to our centre had acute vasodilator response testing performed, most of them with NO and for a minority of them with epoprostenol (12%). Recent ERS/ESC guidelines suggest that NO is the drug of choice for acute testing because of a better safety profile but epoprostenol may be an alternative.8,9 Indeed, it has been clearly demonstrated that haemodynamic acute responses to NO or epoprostenol are broadly similar, suggesting that the drugs used to test acute reactivity may not influence our results.5 Therefore, by choosing this time period, we had the opportunity to analyse data from 663 consecutive PAH patients and long-term follow-up of the acute responders. At this time, an acute vasodilator response was defined by a relative decrease of >20% in both mPAP and vascular resistance, then in the present study, only patients who met these criteria have been treated with CCB. The criteria of decrease in mPAP and TPR used in this study were accurate to screen long-term responders; however, these criteria had low specificity and only 16/43 patients (37.2%) who met the criteria had a long-term response to CCB. In iPAH, we have previously demonstrated that a decrease in mPAP of ≥10 mmHg, reaching an absolute mPAP value of <40 mmHg, associated with no change or an increase in CO during acute testing, was the best predictor of long-term response to CCB.5 When these criteria were applied to our cohort of PAH patients, 29 patients would have had an acute response and 14 of them (48.3%) had a long-term response to CCB. This proportion was lower than previous results in iPAH, except in the subgroup of PAH associated with anorexigen use in which the new criteria had similar specificity to that in iPAH.5 As observed in iPAH, these criteria are more specific to detect long-term responders; thus, 12 patients without a long-term CCB response would not have received potentially harmful CCB. Conversely, 2/16 patients with a long-term response did not meet these criteria during acute testing and would not have received CCB. Interestingly, even if these two patients had a response to CCB at 1 year, this response was not sustained and both experienced clinical and haemodynamic deterioration requiring additional specific PAH therapy in the 5 years following diagnosis. These data confirm the importance of assessing the haemodynamic response to acute vasodilation without being too restrictive in terms of criteria and support close monitoring of these patients, including haemodynamic revaluation after 3 or 4 months and clinical revaluation every year, to confirm the sustained response to CCB.
To conclude, as observed in iPAH, ∼10% of patients with anorexigen-associated PAH may benefit from long-term CCB therapy. These patients can be identified by acute vasodilator testing. Recent criteria, defined by a decrease in mPAP of ≥10 mmHg, reaching an absolute mPAP value of <40 mmHg, associated with no change or an increase in CO during acute testing, are of interest in this subset of patients to predict long-term response to CCB.5 Acute vasodilator testing has questionable value in PAH patients with PVOD/PCH, CHD, or CTD because of the absence of long-term CCB response and the risk of developing pulmonary oedema or clinical deterioration in PVOD/PCH and PAH associated with CTD after initiation of CCB. In contrast, even though it was uncommon, a long-term response to CCB is possible in PAH patients with HIV infection or PoPH. In long-term CCB responders, the prognosis was good (all patients alive at 5 years) and directly related to the underlying cause of PAH.
Acknowledgements
The authors thank Dr L.C. Price and Newmed Publishing for reading the manuscript.
Conflict of interest: none declared.