Abstract
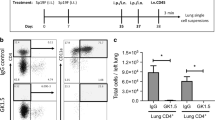
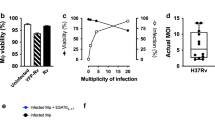
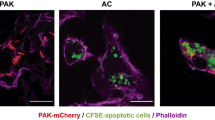
CD1d-restricted T cells are implicated as key players in host defense against various microbial infections. However, the mechanisms involved and the role they play, if any, at the mucosal surfaces where pathogenic infections are initiated is unknown. In a murine pneumonia model established by intranasal application of Pseudomonas aeruginosa, CD1d−/− mice showed markedly reduced pulmonary eradication of P. aeruginosa compared with wild-type mice; this was associated with significantly lower amounts of macrophage inflammatory protein-2 and reduced numbers of neutrophils within the bronchoalveolar lavage fluid. Corollarily, treatment of mice with α-galactosylceramide—a lipid that activates CD1d-restricted T cells—increased the amount of interferon-γ; this was associated with rapid pulmonary clearance through enhanced phagocytosis of P. aeruginosa by alveolar macrophages. These results reveal a crucial role played by CD1d-restricted T cells in regulating the antimicrobial immune functions of macrophages at the lung mucosal surface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Paulnock, D.M. Macrophage activation by T cells. Curr. Opin. Immunol. 4, 344–349 (1992).
Kindler, V., Sappino, A.P., Grau, G.E., Piguet, P.F. & Vassalli, P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56, 731–740 (1989).
Cheung, D.O., Halsey, K. & Speert, D.P. Role of pulmonary alveolar macrophages in defense of the lung against Pseudomonas aeruginosa. Infect. Immun. 68, 4585–4592 (2000).
Hashimoto, S. et al. Depletion of alveolar macrophages decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am. J. Physiol. (Lung Cellular and Molecular Physiology) 270, L819–828 (1996).
Tsai, W.C. et al. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 68, 4289–4296 (2000).
Chen, G.H. et al. Intrapulmonary TNF gene therapy reverses sepsis-induced suppression of lung antibacterial host defense. J. Immunol. 165, 6496–6503 (2000).
Porcelli, S.A. & Modlin, R.L. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17, 297–329 (1999).
Roark, J.H. et al. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J. Immunol. 160, 3121–3127 (1998).
Munoz-Fernandez, M.A., Fernandez, M.A. & Fresno, M. Synergism between tumor necrosis factor-α and interferon-γ on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur. J. Immunol. 22, 301–307 (1992).
Stout, R.D. & Bottomly, K. Antigen-specific activation of effector macrophages by IFN-γ producing (TH1) T cell clones. Failure of IL-4-producing (TH2) T cell clones to activate effector function in macrophages. J. Immunol. 142, 760–765 (1989).
Kumar, H., Belperron, A., Barthold, S.W. & Bockenstedt, L.K. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J. Immunol. 165, 4797–4801 (2000).
Gonzalez-Aseguinolaza, G. et al. α-galactosylceramide-activated Vα14 natural killer T cells mediate protection against murine malaria. Proc. Natl. Acad. Sci. USA 97, 8461–8466 (2000).
Kawakami, K. et al. Activation of Vα14+ natural killer T cells by α- galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans. Infect. Immun. 69, 213–220 (2001).
Exley, M.A. et al. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J. Leukoc. Biol. 69, 713–718 (2001).
Faunce, D.E., Sonoda, K.H. & Stein-Streilein, J. MIP-2 recruits NKT cells to the spleen during tolerance induction. J. Immunol. 166, 313–321 (2001).
Schroeder, T.H. et al. Transgenic cystic fibrosis mice exhibit reduced early clearance of Pseudomonas aeruginosa from the respiratory tract. J. Immunol. 166, 7410–7418 (2001).
Saubermann, L.J. et al. Activation of natural killer T cells by α-galactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology 119, 119–128 (2000).
Vernacchio, L. et al. Comparison of an opsonophagocytic assay and IgG ELISA to assess responses to pneumococcal polysaccharide and pneumococcal conjugate vaccines in children and young adults with sickle cell disease. J. Infect. Dis. 181, 1162–1166 (2000).
Contursi, C. et al. IFN consensus sequence binding protein potentiates STAT1-dependent activation of IFNγ-responsive promoters in macrophages. Proc. Natl. Acad. Sci. USA 97, 91–96 (2000).
Naidenko, O. et al. Binding and antigen presentation of ceramide-containing glycolipids by soluble mouse and human CD1d molecules. J. Exp. Med. 190, 1069–1080 (1999)
Speert, D.P. & Gordon, S. Phagocytosis of unopsonized Pseudomonas aeruginosa by murine macrophages is a two-step process requiring glucose. J. Clin. Invest. 90, 1085–1092 (1992).
Kooguchi K. et al. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect. Immun. 66, 3164–3169 (1998).
Kawakami, K. et al. Monocyte chemoattractant protein-1-dependent increase of Vα14 NKT cells in lungs and their roles in Th1 response and host defense in cryptococcal infection. J. Immunol. 167, 6525–6532 (2001).
Spada, F.M. et al. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur. J. Immunol. 30, 3468–3477 (2000)
Wilson, S.B. & Byrne, M.C. Gene expression in NKT cells: defining a functionally distinct CD1d-restricted T cell subset. Curr. Opin. Immunol. 13, 555–561 (2001)
Smiley, S.T., Kaplan, M.H. & Grusby, M.J. Immunoglobin E production in the absence of interleukin-4 secreting CD1-dependent cells. Science 275, 977–979 (1997).
Sonoda, K.H., Exley, M., Snapper, S., Balk, S.P. & Stein-Streilein, J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-priviledged site. J. Exp. Med. 190, 1215–1226 (1999).
Acknowledgements
We thank S. Shapiro for helpful discussions; I. Wang for technical support; and Kirin Pharmaceuticals for generously providing the glycolipids we used. R.S.B. was supported by NIH grants DK44319, DK51362 and DK53056 and the Harvard Digestive Diseases Center. S.P.C. was supported by grant PO1 DE 13499. E.E.S.N. was supported by the Ter Meulen Fund, Royal Netherlands Academy of Arts and Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nieuwenhuis, E., Matsumoto, T., Exley, M. et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med 8, 588–593 (2002). https://doi.org/10.1038/nm0602-588
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0602-588
This article is cited by
-
Regeneration of invariant natural killer T (iNKT) cells: application of iPSC technology for iNKT cell-targeted tumor immunotherapy
Inflammation and Regeneration (2023)
-
Innate immune responses in pneumonia
Pneumonia (2023)
-
Regulation of invariant NKT cell development and function by a 0.14 Mbp locus on chromosome 1: a possible role for Fcgr3
Genes & Immunity (2019)
-
Generation of a Jurkat-based fluorescent reporter cell line to evaluate lipid antigen interaction with the human iNKT cell receptor
Scientific Reports (2019)
-
Genetically Diversity of Pseudomonas aeruginosa Isolated from Chronic Suppurative Otitis Media with Respect to Their Antibiotic Sensitivity Pattern
Indian Journal of Otolaryngology and Head & Neck Surgery (2019)