Abstract
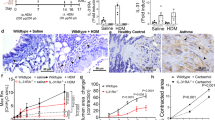
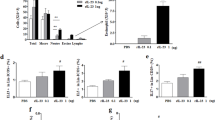
Emerging evidence suggests that the T helper 17 (TH17) subset of αβ T cells contributes to the development of allergic asthma. In this study, we found that mice lacking the αvβ8 integrin on dendritic cells did not generate TH17 cells in the lung and were protected from airway hyper-responsiveness in response to house dust mite and ovalbumin sensitization and challenge. Because loss of TH17 cells inhibited airway narrowing without any obvious effects on airway inflammation or epithelial morphology, we examined the direct effects of TH17 cytokines on mouse and human airway smooth muscle function. Interleukin-17A (IL-17A), but not IL-17F or IL-22, enhanced contractile force generation of airway smooth muscle through an IL-17 receptor A (IL-17RA)–IL-17RC, nuclear factor κ light-chain enhancer of activated B cells (NF-κB)–ras homolog gene family, member A (RhoA)–Rho-associated coiled-coil containing protein kinase 2 (ROCK2) signaling cascade. Mice lacking integrin αvβ8 on dendritic cells showed impaired activation of this pathway after ovalbumin sensitization and challenge, and the diminished contraction of the tracheal rings in these mice was reversed by IL-17A. These data indicate that the IL-17A produced by TH17 cells contributes to allergen-induced airway hyper-responsiveness through direct effects on airway smooth muscle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lloyd, C.M. & Hessel, E.M. Functions of T cells in asthma: more than just T(H)2 cells. Nat. Rev. Immunol. 10, 838–848 (2010).
Grünig, G. et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282, 2261–2263 (1998).
Alcorn, J.F., Crowe, C.R. & Kolls, J.K. TH17 cells in asthma and COPD. Annu. Rev. Physiol. 72, 495–516 (2010).
Souwer, Y., Szegedi, K., Kapsenberg, M.L. & de Jong, E.C. IL-17 and IL-22 in atopic allergic disease. Curr. Opin. Immunol. 22, 821–826 (2010).
Lajoie, S. et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat. Immunol. 11, 928–935 (2010).
Littman, D.R. & Rudensky, A.Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140, 845–858 (2010).
Finkelman, F.D., Hogan, S.P., Hershey, G.K., Rothenberg, M.E. & Wills-Karp, M. Importance of cytokines in murine allergic airway disease and human asthma. J. Immunol. 184, 1663–1674 (2010).
Bullens, D.M. et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir. Res. 7, 135 (2006).
de Faria, I.C., de Faria, E.J., Toro, A.A., Ribeiro, J.D. & Bertuzzo, C.S. Association of TGF-β1, CD14, IL-4, IL-4R and ADAM33 gene polymorphisms with asthma severity in children and adolescents. J. Pediatr. (Rio J.) 84, 203–210 (2008).
Pulleyn, L.J., Newton, R., Adcock, I.M. & Barnes, P.J. TGFβ1 allele association with asthma severity. Hum. Genet. 109, 623–627 (2001).
Silverman, E.S. et al. Transforming growth factor-β1 promoter polymorphism C-509T is associated with asthma. Am. J. Respir. Crit. Care Med. 169, 214–219 (2004).
Zeyrek, D. et al. Association of interleukin-1β and interleukin-1 receptor antagonist gene polymorphisms in Turkish children with atopic asthma. Allergy Asthma Proc. 29, 468–474 (2008).
Settin, A., Zedan, M., Farag, M., Ezz El Regal, M. & Osman, E. Gene polymorphisms of IL-6(-174) G/C and IL-1Ra VNTR in asthmatic children. Indian J. Pediatr. 75, 1019–1023 (2008).
Mangan, P.R. et al. Transforming growth factor-β induces development of the T(H)17 lineage. Nature 441, 231–234 (2006).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Ivanov, I.I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Yang, X.O. et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282, 9358–9363 (2007).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007).
Atarashi, K. et al. ATP drives lamina propria TH17 cell differentiation. Nature 455, 808–812 (2008).
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell–mediated autoimmunity to environmental toxins. Nature 453, 106–109 (2008).
Ivanov, I.I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Manel, N., Unutmaz, D. & Littman, D.R. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat. Immunol. 9, 641–649 (2008).
Volpe, E. et al. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nat. Immunol. 9, 650–657 (2008).
Annes, J.P., Munger, J.S. & Rifkin, D.B. Making sense of latent TGFβ activation. J. Cell Sci. 116, 217–224 (2003).
Melton, A.C. et al. Expression of αvβ8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J. Clin. Invest. 120, 4436–4444 (2010).
Travis, M.A. et al. Loss of integrin α(v) β8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449, 361–365 (2007).
Fossiez, F. et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183, 2593–2603 (1996).
Kolls, J.K. & Linden, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004).
Blaho, V.A., Buczynski, M.W., Dennis, E.A. & Brown, C.R. Cyclooxygenase-1 orchestrates germinal center formation and antibody class-switch via regulation of IL-17. J. Immunol. 183, 5644–5653 (2009).
Lin, Y. et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31, 799–810 (2009).
Wills-Karp, M. et al. Interleukin-13: central mediator of allergic asthma. Science 282, 2258–2261 (1998).
Tliba, O. et al. IL-13 enhances agonist-evoked calcium signals and contractile responses in airway smooth muscle. Br. J. Pharmacol. 140, 1159–1162 (2003).
Parris, J.R., Cobban, H.J., Littlejohn, A.F., MacEwan, D.J. & Nixon, G.F. Tumour necrosis factor-α activates a calcium sensitization pathway in guinea-pig bronchial smooth muscle. J. Physiol. (Lond.) 518, 561–569 (1999).
Barnes, P.J., Cuss, F.M. & Palmer, J.B. The effect of airway epithelium on smooth muscle contractility in bovine trachea. Br. J. Pharmacol. 86, 685–691 (1985).
Barnett, K., Jacoby, D.B., Nadel, J.A. & Lazarus, S.C. The effects of epithelial cell supernatant on contractions of isolated canine tracheal smooth muscle. Am. Rev. Respir. Dis. 138, 780–783 (1988).
Somlyo, A.P. & Somlyo, A.V. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. (Lond.) 522, 177–185 (2000).
Sonder, S.U. et al. IL-17-induced NF-κB activation via CIKS/Act1: physiologic significance and signaling mechanisms. J. Biol. Chem. 286, 12881–12890 (2011).
Yao, Z. et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3, 811–821 (1995).
Goto, K. et al. The proximal STAT6 and NF-κB sites are responsible for IL-13– and TNF-α–induced RhoA transcriptions in human bronchial smooth muscle cells. Pharmacol. Res. 61, 466–472 (2010).
Shimada, H. & Rajagopalan, L.E. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-κB p65. J. Biol. Chem. 285, 12536–12542 (2010).
Chiba, Y. & Misawa, M. The role of RhoA-mediated Ca2+ sensitization of bronchial smooth muscle contraction in airway hyperresponsiveness. J. Smooth Muscle Res. 40, 155–167 (2004).
Khromov, A., Choudhury, N., Stevenson, A.S., Somlyo, A.V. & Eto, M. Phosphorylation-dependent autoinhibition of myosin light chain phosphatase accounts for Ca2+ sensitization force of smooth muscle contraction. J. Biol. Chem. 284, 21569–21579 (2009).
Ely, L.K., Fischer, S. & Garcia, K.C. Structural basis of receptor sharing by interleukin 17 cytokines. Nat. Immunol. 10, 1245–1251 (2009).
Patil, S.B. & Bitar, K.N. RhoA- and PKC-α–mediated phosphorylation of MYPT and its association with HSP27 in colonic smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G83–G95 (2006).
Ohama, T., Hori, M., Sato, K., Ozaki, H. & Karaki, H. Chronic treatment with interleukin-1β attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J. Biol. Chem. 278, 48794–48804 (2003).
Chen, X.Y., Dun, J.N., Miao, Q.F. & Zhang, Y.J. Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, suppresses 5-hydroxytryptamine–induced pulmonary artery smooth muscle cell proliferation via JNK and ERK1/2 pathway. Pharmacology 83, 67–79 (2009).
McKinley, L. et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J. Immunol. 181, 4089–4097 (2008).
Schnyder-Candrian, S. et al. Interleukin-17 is a negative regulator of established allergic asthma. J. Exp. Med. 203, 2715–2725 (2006).
Nakae, S. et al. Antigen-specific T cell sensitization is impaired in IL-17–deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387 (2002).
Wilson, R.H. et al. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am. J. Respir. Crit. Care Med. 180, 720–730 (2009).
Oda, N. et al. Interleukin-17F induces pulmonary neutrophilia and amplifies antigen-induced allergic response. Am. J. Respir. Crit. Care Med. 171, 12–18 (2005).
Hellings, P.W. et al. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am. J. Respir. Cell Mol. Biol. 28, 42–50 (2003).
Song, C. et al. IL-17–producing alveolar macrophages mediate allergic lung inflammation related to asthma. J. Immunol. 181, 6117–6124 (2008).
Willis-Karp, M. et al. Interleukin-13: central mediator of allergic asthma. Science 282, 2258–2261 (1998).
Grünig, G. et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282, 2261–2263 (1998).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
Bai, Y. & Sanderson, M.J. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L208–L221 (2006).
Acknowledgements
This work was supported by US National Institutes of Health (NIH) grants HL64353, HL53949, HL083950, AI024674 and U19 AI077439 (to D.S.), a NIH Ruth L. Kirschstein National Research Service Award HL095314 (to A.C.M.), an American Lung Association of California Research Training Fellowship Award (to A.C.M.) and funds from the University of California, San Francisco (UCSF) Strategic Asthma Basic Research Center. Deidentified human lung was kindly provided by M. Matthay (UCSF) and P. Wolters (UCSF). IL-17RC knockout mice were kindly provided by W. Ouyang (Genentech).
Author information
Authors and Affiliations
Contributions
M.K. designed the study, generated all of the tracheal ring contraction and western blot data, performed analyses and wrote the manuscript. A.C.M. designed the study, generated all of the flow cytometry and recall data, performed analyses and wrote the manuscript. C.C. generated all of the lung slice data, performed analyses and wrote the manuscript. K.E.H., X.R., Y.W. and X.B. performed the asthma physiology studies. M.B.E. designed and constructed the muscle bath and provided expertise in the methodology and modifications for the analysis of mouse tracheal ring contraction. J.T.L. and K.A. provided substantial intellectual contribution. X.H. and D.S. oversaw the design and interpretation of all studies described and oversaw the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 520 kb)
Rights and permissions
About this article
Cite this article
Kudo, M., Melton, A., Chen, C. et al. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 18, 547–554 (2012). https://doi.org/10.1038/nm.2684
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2684
This article is cited by
-
RORγt inhibitors block both IL-17 and IL-22 conferring a potential advantage over anti-IL-17 alone to treat severe asthma
Respiratory Research (2021)
-
Diesel exhaust particles increase nasal symptoms and IL-17A in house dust mite-induced allergic mice
Scientific Reports (2021)
-
The role of peripheral type 2 innate lymphoid cells in bronchiolitis
Scientific Reports (2021)
-
Pentraxin 3 promotes airway inflammation in experimental asthma
Respiratory Research (2020)
-
Cytokine-induced molecular responses in airway smooth muscle cells inform genome-wide association studies of asthma
Genome Medicine (2020)