Abstract
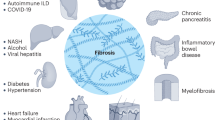
Fibrocytes are cells that circulate in the peripheral blood and produce connective tissue proteins such as vimentin and collagens I and III. Fibrocytes are associated with skin lesions, pulmonary fibrosis, and tumors and they contribute to the remodeling response by secreting matrix metalloproteinases. Fibrocytes can further differentiate, and they are a likely source of the contractile myofibroblast that appears in many fibrotic lesions. There is evidence in the skin for a prominent role for fibrocytes in the development of hypertrophic scars and keloids. In asthma or in experimental models of pulmonary fibrosis, fibrocytes have been shown to infiltrate areas of inflammation and tissue damage. Fibrocytes constitute part of the stromal response to tumor invasion, and there is evidence that these cells may be a prognosticator of malignant potential. IL-1, TGF-β, chemokines, and serum amyloid P modulate the appearance and function of fibrocytes. Fibrocytes themselves produce inflammatory cytokines, growth factors, and chemokines. The intercellular signals that modulate fibrocyte trafficking, proliferation, and differentiation are only partially defined, but a better understanding of these signals enable new therapies to prevent pathologic fibrosis or to improve the tissue repair response.
Similar content being viewed by others
References and Recommended Reading
Chesney J, Bucala R: Peripheral blood fibrocytes. In In Human Cell Culture. Edited by Pallsson BO, Koller MR, Masters J. London: Kluwer Academic Publishers; 2001:209–219.
Bucala R, Spiegel LA, Chesney J, et al.: Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1994, 1:71–81. First description of circulating fibrocytes.
Burkitt Y, Heath H: Wheater’s Functional Histology, vol 1, edn 3. London: Churchill Livingston; 1997.
Schmidt M, Sun G, Stacey MA, et al.: Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003, 71:380–389. First report of fibrocytes in human lung disease. Cells were identified in asthma patients.
Mori L, Bellini A, Stacey MA, et al.: Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res 2005, 304:81–90.
Fathke C, Wilson L, Hutter J, et al.: Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 2004, 22:812–822.
Hashimoto N, Jin H, Liu T, et al.: Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004, 113:243–252.
Opalenik SR, Davidson JM: Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J 2005, 19:1561–1563.
Abe R, Donnelly SC, Peng T, et al.: Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001, 166:7556–7562. First identification of trafficking signals of fibrocytes in vivo.
Yang L, Scott PG, Giuffre J, et al.: Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest 2002, 82:1183–1192.
Labat ML, Bringuier AF, Arys-Philippart C, et al.: Monocytic origin of fibrosis. In vitro transformation of HLA-DR monocytes into neo-fibroblasts: inhibitory effect of all-trans retinoic acid on this process. Biomed Pharmacother 1994, 48:103–111.
Moore BB, Kolodsick JE, Thannickal VJ, et al.: CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 2005, 166:675–684.
Phillips RJ, et al.: Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004, 114(3):p. 438–46.
Chesney J, Metz C, Stavitsky AB, et al.: Regulated production of type I collagen and inf lammator y cytokines by peripheral blood fibrocytes. J Immunol 1998, 160:419–425. Characterization of fibrocyte phenotype and effector function. Described fibrocytes in granuloma.
Robson RT, Smith DJ: Wounds and wound healing. In Essentials of Genereal Surgery. Edited by Lawrence P, Satterfield T. Baltimore: Williams and Wilkins; 1992:119–125.
Aiba S, Tagami H: Inverse correlation between CD34 expression and proline-4-hydroxylase immunoreactivity on spindle cells noted in hypertrophic scars and keloids. J Cutan Pathol 1997, 24:65–69.
Yang L, Scott PG, Dodd C, et al.: Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen 2005, 13:398–404.
Hartlapp I, Abe R, Saeed RW, et al.: Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J 2001, 15:2215–2224.
Barth PJ, Ebrahimsade S, Ramaswamy A, Moll R: CD34+ fibrocytes in invasive ductal carcinoma, ductal carcinoma in situ, and benign breast lesions. Virchows Arch 2002, 440:298–303.
Chauhan H, Abraham A, Phillips JR, et al.: There is more than one kind of myofibroblast: analysis of CD34 expression in benign, in situ, and invasive breast lesions. J Clin Pathol 2003, 56:271–276.
Ramaswamy A, Moll R, Barth PJ: CD34+ fibrocytes in tubular carcinomas and radial scars of the breast. Virchows Arch 2003, 443:536–540.
Barth PJ, Ebrahimsade S, Hellinger A, et al.: CD34+ fibrocytes in neoplastic and inflammatory pancreatic lesions. Virchows Arch 2002, 440:128–133.
Barth PJ, Ramaswamy A, Moll R: CD34(+) fibrocytes in normal cer vical stroma, cer vical intraepithelial neoplasia III, and invasive squamous cell carcinoma of the cer vix uteri. Virchows Arch 2002, 441:564–568.
Kirchmann TT, Prieto VG, Smoller BR: CD34 staining pattern distinguishes basal cell carcinoma from trichoepithelioma. Arch Dermatol 1994, 130:589–592.
Barth PJ, Schenckzu Schweinsberg T, Ramaswamy A, Moll R, et al.: CD34+ fibrocytes, alpha-smooth muscle antigen-positive myoflbroblasts, and CD117 expression in the stroma of invasive squamous cell carcinomas of the oral cavity, pharynx, and larynx. Virchows Arch 2004, 444:231–234.
Humphreys TR, Monteiro MR, Murphy GF: Mast cells and dendritic cells in basal cell carcinoma stroma. Dermatol Surg 2000, 26:200–203.
Kirchmann TT, Prieto VG, Smoller BR: Use of CD34 in assessing the relationship between stroma and tumor in desmoplastic keratinocytic neoplasms. J Cutan Pathol 1995, 22:422–426.
Aiba S, et al.: CD34+ spindle-shaped cells selectively disappear from the skin lesion of scleroderma. Arch Dermatol 1994, 130(5):p. 593–7.
White B, et al.: Systemic sclerosis and related syndromes a epidemiology, pathology and pathogenesis. In Primer on the Rheumatic Diseases. Edited by Klippel J. Atlanta: Arthritis Foundation; 2001:354–357.
Pilling D, Buckley CD, Salmon M, Gomer RH: Inhibition of.brocyte differentiation by serum amyloid P. J Immunol 2003, 171:5537–5546. Interesting report linking SAP with fibrocyte differentiation and potential role in fibrosing disorder such as scleroderma.
Cowper SE, Su LD, Bhawan J, et al.: Nephrogenic fibrosing dermopathy. Am J Dermatopathol 2001, 23:383–393. First clinical description of NFD in patients.
Nephrogenic Fibrosing Dermopathy[NFD website]. 2001- present, Cowper SE. http:/ www.pathmax.com/dermweb. Accessed December 1, 2005. National Registry for NFD.
Moschella SL, Kay J, Mackool BT, Liu V: Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 35-2004. A 68-year-old man with end-stage renal disease and thickening of the skin. N Engl J Med 2004, 351:2219–2227.
Cowper SE: Nephrogenic fibrosing dermopathy: the first 6 years. Curr Opin Rheumatol 2003, 15:785–790.
Cowper SE, Bucala R: Nephrogenic fibrosing dermopathy: suspect identified, motive unclear. Am J Dermatopathol 2003, 25:358.
Savage DD, Garrison RJ, Devereux RB, et al.: Mitral valve prolapse in the general population. 1. Epidemiologic features: the Framingham Study. Am Heart J 1983, 106:571–576.
Freed L A, Levy D, Levine R A, et al.: Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999, 341:1–7.
Barth PJ, Koster H, Moosdorf R: CD34+ fibrocytes in normal mitral valves and myxomatous mitral valve degeneration. Pathol Res Pract 2005, 201:301–304.
Paget J: Lectures on Surgical Pathology. In Royal College of Surgeons of England. London: Longmans; 1863.
Stirling GA, Kakkar V V: Cells in the circulating blood capable of producing connective tissue. Br J Exp Pathol 1969, 50:51–55.
Cowper SE, Bucala R: Nephrogenic fibrosing dermopathy: suspect identified, motive unclear. Am J Dermatopathol 2003, 25:358.
Direkze NC, Hodivala-Dilke K, Jeffery R, et al.: Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res 2004, 64:8492–8495.
Nagy N, Biro E, Takacs A, et al.: Peripheral blood fibrocytes contribute to the formation of the avian spleen. Dev Dyn 2005, 232:55–66.
Hong KM, Burdick MD, Phillips RJ, et al.: Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J 2005, 19:2029–2031.
Chesney J, Bacher M, Bender A, et al.: The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A 1997, 94:6307–6312.
Balmelli C, Ruggli N, McCullough K, Summerfield A, et al.:Fibrocytes are potent stimulators of anti-virus cytotoxic T cells. J Leukoc Biol 2005, 77:923–933.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quan, T.E., Cowper, S.E. & Bucala, R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep 8, 145–150 (2006). https://doi.org/10.1007/s11926-006-0055-x
Issue Date:
DOI: https://doi.org/10.1007/s11926-006-0055-x