Abstract
Purpose
Individual clinical courses of idiopathic interstitial pneumonia (IIP) are variable and difficult to predict because the pathology and disease activity are contingent, and chest computed tomography (CT) provides little information about disease activity. In this study, we applied dual-time-point [18F]-fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography (PET), commonly used for diagnosis of malignant tumours, to the differential diagnosis and prediction of disease progression in IIP patients.
Methods
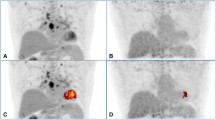
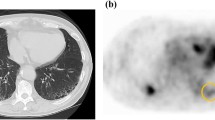
Fifty patients with IIP, including idiopathic pulmonary fibrosis (IPF, n = 21), non-specific interstitial pneumonia (NSIP, n = 18) and cryptogenic organizing pneumonia (COP, n = 11), underwent 18F-FDG PET examinations at two time points: scan 1 at 60 min (early imaging) and scan 2 at 180 min (delayed imaging) after 18F-FDG injection. The standardized uptake values (SUV) at the two points and the retention index (RI-SUV) calculated from them were evaluated and compared with chest CT findings, disease progression and disease types. To evaluate short-term disease progression, all patients were examined by pulmonary function test every 3 months for 1 year after 18F-FDG PET scanning.
Results
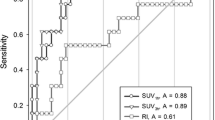
The early SUV for COP (2.47 ± 0.74) was significantly higher than that for IPF (0.99 ± 0.29, p = 0.0002) or NSIP (1.22 ± 0.44, p= 0.0025). When an early SUV cut-off value of 1.5 and greater was used to distinguish COP from IPF and NSIP, the sensitivity, specificity and accuracy were 90.9, 94.3 and 93.5%, respectively. The RI-SUV for IPF and NSIP lesions was significantly greater in patients with deteriorated pulmonary function after 1 year of follow-up (progressive group, 13.0 ± 8.9%) than in cases without deterioration during the 1-year observation period (stable group, −16.8 ± 5.9%, p < 0.0001). However, the early SUV for all IIP types provided no additional information of disease progression. When an RI-SUV cut-off value of 0% and greater was used to distinguish progressive IIPs from stable IIPs, the sensitivity, specificity and accuracy were 95.5, 100 and 97.8%, respectively.
Conclusion
Early SUV and RI-SUV obtained from dual-time-point 18F-FDG PET are useful parameters for the differential diagnosis and prediction of disease progression in patients with IIP.





Similar content being viewed by others
References
American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277–304.
Park JH, Kim DK, Kim DS, Koh Y, Lee SD, Kim WS, et al. Mortality and risk factors for surgical lung biopsy in patients with idiopathic interstitial pneumonia. Eur J Cardiothorac Surg 2007;31:1115–9. doi:10.1016/j.ejcts.2007.02.035.
Utz JP, Ryu JH, Douglas WW, Hartman TE, Tazelaar HD, Myers JL, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J 2001;17:175–9. doi:10.1183/09031936.01.17201750.
Monaghan H, Wells AU, Colby TV, du Bois RM, Hansell DM, Nicholson AG. Prognostic implications of histologic patterns in multiple surgical lung biopsies from patients with idiopathic interstitial pneumonias. Chest 2004;125:522–6. doi:10.1378/chest.125.2.522.
Demura Y, Tsuchida T, Ishizaki T, Mizuno S, Totani Y, Ameshima S, et al. 18F-FDG accumulation with PET for differentiation between benign and malignant lesions in the thorax. J Nucl Med 2003;44:540–8.
Shin L, Katz DS, Yung E. Hypermetabolism on F-18 FDG PET of multiple pulmonary nodules resulting from bronchiolitis obliterans organizing pneumonia. Clin Nucl Med 2004;29:654–6. doi:10.1097/00003072-200410000-00017.
Meissner HH, Soo Hoo GW, Khonsary SA, Mandelkern M, Brown CV, Santiago SM. Idiopathic pulmonary fibrosis: evaluation with positron emission tomography. Respiration 2006;73:197–202.
Morikawa M, Demura Y, Mizuno S, Ameshima S, Ishizaki T, Okazawa H. FDG positron emission tomography imaging of drug-induced pneumonitis. Ann Nucl Med 2008;22:335–8. doi:10.1007/s12149-007-0109-9.
Kim IJ, Lee JS, Kim SJ, Kim YK, Jeong YJ, Jun S, et al. Double-phase 18F-FDG PET-CT for determination of pulmonary tuberculoma activity. Eur J Nucl Med Mol Imaging 2008;35:808–14. doi:10.1007/s00259-007-0585-0.
Deichen JT, Prante O, Gack M, Schmiedehausen K, Kuwert T. Uptake of [18F]fluorodeoxyglucose in human monocyte-macrophages in vitro. Eur J Nucl Med Mol Imaging 2003;30:267–73.
Ishimori T, Saga T, Mamede M, Kobayashi H, Higashi T, Nakamoto Y, et al. Increased (18)F-FDG uptake in a model of inflammation: concanavalin A-mediated lymphocyte activation. J Nucl Med 2002;43:658–63.
Schuster DP, Brody SL, Zhou Z, Bernstein M, Arch R, Link D, et al. Regulation of lipopolysaccharide-induced increases in neutrophil glucose uptake. Am J Physiol Lung Cell Mol Physiol 2007;292:L845–51. doi:10.1152/ajplung.00350.2006.
Watters LC, King TE, Schwarz MI, Waldron JA, Stanford RE, Cherniack RM. A clinical, radiographic, and physiologic scoring system for the longitudinal assessment of patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis 1986;133:97–103.
Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al.. General considerations for lung function testing. Eur Respir J 2005;26:153–61. doi:10.1183/09031936.05.00034505.
American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646–64.
Nagai S, Kitaichi M, Itoh H, Izumi T, Colby TV. Idiopathic nonspecific interstitial pneumonia/fibrosis: comparison with idiopathic pulmonary fibrosis and BOOP. Eur Respir J 1998;12:1010–9. doi:10.1183/09031936.98.12051010.
Kim DS, Collard HR, King TE Jr. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006;3:285–92. doi:10.1513/pats.200601-005TK.
Travis WD, Matsui K, Moss J, Ferrans VJ. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol 2000;24:19–33. doi:10.1097/00000478-200001000-00003.
Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 2000;162:2213–7.
Collard HR, King TE Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003;168:538–42. doi:10.1164/rccm.200211-1311OC.
Flaherty KR, Mumford JA, Murray S, Kazerooni EA, Gross BH, Colby TV, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003;168:543–8. doi:10.1164/rccm.200209-1112OC.
Jegal Y, Kim DS, Shim TS, Lim CM, Do Lee S, Koh Y, et al. Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 2005;171:639–44. doi:10.1164/rccm.200403-331OC.
Latsi PI, du Bois RM, Nicholson AG, Colby TV, Bisirtzoglou D, Nikolakopoulou A, et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med 2003;168:531–7. doi:10.1164/rccm.200210-1245OC.
Hamberg LM, Hunter GJ, Alpert NM, Choi NC, Babich JW, Fischman AJ. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med 1994;35:1308–12.
Hustinx R, Smith RJ, Benard F, Rosenthal DI, Machtay M, Farber LA, et al. Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from inflammation and normal tissue in the head and neck. Eur J Nucl Med 1999;26:1345–8. doi:10.1007/s002590050593.
Acknowledgments
We thank Dr. Norihiro Naiki (Department of Molecular Pathology, University of Fukui) for valuable advice regarding pathological diagnosis.
This work was supported by the 21st Century COE program “Biomedical Imaging Technology Integration Program” from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umeda, Y., Demura, Y., Ishizaki, T. et al. Dual-time-point 18F-FDG PET imaging for diagnosis of disease type and disease activity in patients with idiopathic interstitial pneumonia. Eur J Nucl Med Mol Imaging 36, 1121–1130 (2009). https://doi.org/10.1007/s00259-009-1069-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1069-1